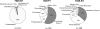Subsurface microbial diversity in deep-granitic-fracture water in Colorado - PubMed (original) (raw)
Subsurface microbial diversity in deep-granitic-fracture water in Colorado
Jason W Sahl et al. Appl Environ Microbiol. 2008 Jan.
Abstract
A microbial community analysis using 16S rRNA gene sequencing was performed on borehole water and a granite rock core from Henderson Mine, a >1,000-meter-deep molybdenum mine near Empire, CO. Chemical analysis of borehole water at two separate depths (1,044 m and 1,004 m below the mine entrance) suggests that a sharp chemical gradient exists, likely from the mixing of two distinct subsurface fluids, one metal rich and one relatively dilute; this has created unique niches for microorganisms. The microbial community analyzed from filtered, oxic borehole water indicated an abundance of sequences from iron-oxidizing bacteria (Gallionella spp.) and was compared to the community from the same borehole after 2 weeks of being plugged with an expandable packer. Statistical analyses with UniFrac revealed a significant shift in community structure following the addition of the packer. Phospholipid fatty acid (PLFA) analysis suggested that Nitrosomonadales dominated the oxic borehole, while PLFAs indicative of anaerobic bacteria were most abundant in the samples from the plugged borehole. Microbial sequences were represented primarily by Firmicutes, Proteobacteria, and a lineage of sequences which did not group with any identified bacterial division; phylogenetic analyses confirmed the presence of a novel candidate division. This "Henderson candidate division" dominated the clone libraries from the dilute anoxic fluids. Sequences obtained from the granitic rock core (1,740 m below the surface) were represented by the divisions Proteobacteria (primarily the family Ralstoniaceae) and Firmicutes. Sequences grouping within Ralstoniaceae were also found in the clone libraries from metal-rich fluids yet were absent in more dilute fluids. Lineage-specific comparisons, combined with phylogenetic statistical analyses, show that geochemical variance has an important effect on microbial community structure in deep, subsurface systems.
Figures
FIG. 1.
Schematic diagram of the Henderson Mine, showing the two levels where water samples were taken (7150 and 7025) as well as the location of the rock core sample.
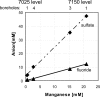
FIG. 2.
Concentrations of manganese, fluoride, and sulfate in water sampled from boreholes at the 7150 and 7025 levels in the Henderson mine.
FIG. 3.
Phylogenetic tree showing representative bacterial divisions constructed with Bayesian inference. Sequences that form the Henderson group 1 are shown in bold. Numbers at bifurcations indicate posterior probabilities calculated by MrBayes v3.1. The rooting archaeal outgroup (a sequence from Halobacteria) has been removed for clarity.
FIG. 4.
Pie charts showing microbial community compositions of drill hole 1 (pre- and postpacker) and drill hole 4 (postpacker), all at the 7025 level. “Others” refers to pooled divisions representing <1% of each respective library.
FIG. 5.
Phylogenetic tree of the division Proteobacteria. Numbers at nodes represent posterior probabilities. Numbers following accession numbers indicate how many sequences grouped with each phylotype.
FIG. 6.
Phylogenetic trees of the division Firmicutes (A) and the domain Archaea (B). Sequences obtained in this study are in bold. Numbers at branching nodes indicate posterior probabilities. The rooting outgroup for the Firmicutes has been removed for clarity.
Similar articles
- Spatial and Temporal Constraints on the Composition of Microbial Communities in Subsurface Boreholes of the Edgar Experimental Mine.
Thieringer PH, Honeyman AS, Spear JR. Thieringer PH, et al. Microbiol Spectr. 2021 Dec 22;9(3):e0063121. doi: 10.1128/Spectrum.00631-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756066 Free PMC article. - Stability of Microbial Community Profiles Associated with Compacted Bentonite from the Grimsel Underground Research Laboratory.
Engel K, Ford SE, Coyotzi S, McKelvie J, Diomidis N, Slater G, Neufeld JD. Engel K, et al. mSphere. 2019 Dec 18;4(6):e00601-19. doi: 10.1128/mSphere.00601-19. mSphere. 2019. PMID: 31852805 Free PMC article. - Bacterial diversity in a mine water treatment plant.
Heinzel E, Hedrich S, Janneck E, Glombitza F, Seifert J, Schlömann M. Heinzel E, et al. Appl Environ Microbiol. 2009 Feb;75(3):858-61. doi: 10.1128/AEM.01045-08. Epub 2008 Dec 1. Appl Environ Microbiol. 2009. PMID: 19047391 Free PMC article. - Microbial communities in subpermafrost saline fracture water at the Lupin Au mine, Nunavut, Canada.
Onstott TC, McGown DJ, Bakermans C, Ruskeeniemi T, Ahonen L, Telling J, Soffientino B, Pfiffner SM, Sherwood-Lollar B, Frape S, Stotler R, Johnson EJ, Vishnivetskaya TA, Rothmel R, Pratt LM. Onstott TC, et al. Microb Ecol. 2009 Nov;58(4):786-807. doi: 10.1007/s00248-009-9553-5. Epub 2009 Jul 1. Microb Ecol. 2009. PMID: 19568805 - Molecular analysis of prokaryotic diversity in the deep subsurface of the former Homestake gold mine, South Dakota, USA.
Rastogi G, Stetler LD, Peyton BM, Sani RK. Rastogi G, et al. J Microbiol. 2009 Aug;47(4):371-84. doi: 10.1007/s12275-008-0249-1. Epub 2009 Sep 9. J Microbiol. 2009. PMID: 19763410
Cited by
- Microbial diversity in the deep-subsurface hydrothermal aquifer feeding the giant gypsum crystal-bearing Naica Mine, Mexico.
Ragon M, Van Driessche AE, García-Ruíz JM, Moreira D, López-García P. Ragon M, et al. Front Microbiol. 2013 Mar 6;4:37. doi: 10.3389/fmicb.2013.00037. eCollection 2013. Front Microbiol. 2013. PMID: 23508882 Free PMC article. - Community structure of subsurface biofilms in the thermal sulfidic caves ofAcquasanta Terme, Italy.
Jones DS, Tobler DJ, Schaperdoth I, Mainiero M, Macalady JL. Jones DS, et al. Appl Environ Microbiol. 2010 Sep;76(17):5902-10. doi: 10.1128/AEM.00647-10. Epub 2010 Jul 16. Appl Environ Microbiol. 2010. PMID: 20639361 Free PMC article. - Paleo-Rock-Hosted Life on Earth and the Search on Mars: A Review and Strategy for Exploration.
Onstott TC, Ehlmann BL, Sapers H, Coleman M, Ivarsson M, Marlow JJ, Neubeck A, Niles P. Onstott TC, et al. Astrobiology. 2019 Oct;19(10):1230-1262. doi: 10.1089/ast.2018.1960. Epub 2019 Jun 25. Astrobiology. 2019. PMID: 31237436 Free PMC article. Review. - Fe-oxide grain coatings support bacterial Fe-reducing metabolisms in 1.7-2.0 km-deep subsurface quartz arenite sandstone reservoirs of the Illinois Basin (USA).
Dong Y, Sanford RA, Locke RA 2nd, Cann IK, Mackie RI, Fouke BW. Dong Y, et al. Front Microbiol. 2014 Sep 30;5:511. doi: 10.3389/fmicb.2014.00511. eCollection 2014. Front Microbiol. 2014. PMID: 25324834 Free PMC article. - Exploration of deep terrestrial subsurface microbiome in Late Cretaceous Deccan traps and underlying Archean basement, India.
Dutta A, Dutta Gupta S, Gupta A, Sarkar J, Roy S, Mukherjee A, Sar P. Dutta A, et al. Sci Rep. 2018 Nov 29;8(1):17459. doi: 10.1038/s41598-018-35940-0. Sci Rep. 2018. PMID: 30498254 Free PMC article.
References
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
- Antweiler, R. C., R. L. Smith, M. A. Voytek, J. K. Bohlke, and K. D. Richards. 2004. Water-quality data from two agricultural drainage basins in the northwestern Indiana and northeastern Illinois. I. Lagrangian and synoptic data, 1999-2002. U.S. Geological Survey Open-File Report 2004-1317. U.S. Geological Survey, Reston, VA.
- Battaglia-Brunet, F., C. Joulian, F. Garrido, M.-C. Dictor, D. Morin, K. Coupland, D. B. Johnson, K. B. Hallberg, and P. Baranger. 2005. Oxidation of arsenite by Thiomonas strains and characterization of Thiomonas arsenivorans sp. nov. Antoine Leeuwenhoek 89:99-108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous