Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow - PubMed (original) (raw)
Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow
Doyon Won et al. Am J Pathol. 2007 Nov.
Abstract
Atherosclerosis develops in distinct regions of the arterial tree. Defining patterns and mechanisms of endothelial cell gene expression in different regions of normal arteries is key to understanding the initial molecular events in atherogenesis. In this study, we demonstrated that the expression of endothelial nitric-oxide synthase (eNOS), an atheroprotective gene, and its phosphorylation on Ser(1177), a marker of activity, were lower in regions of the normal mouse aorta that are predisposed to atherosclerosis. The same expression pattern was observed in mouse strains that are both susceptible and resistant to atherosclerosis, and the topography of eNOS expression was inverse to p65, the main nuclear factor-kappaB subunit. Modeling of disturbed and uniform laminar flow in vitro reproduced the expression patterns of eNOS and p65 that were found in vivo. Heterogeneous nuclear RNA expression and RNA polymerase II chromosome immunoprecipitation studies demonstrated that regulation of transcription contributed to increased eNOS expression in response to shear stress. In vivo, the transcription of eNOS was reduced in regions of the mouse aorta predisposed to atherosclerosis, as defined by reporter gene expression in eNOS promoter-beta-galactosidase reporter transgenic mice. These data suggest that disturbed hemodynamic patterns found at arterial branches and curvatures uniquely modulate endothelial cell gene expression by regulating transcription, potentially explaining why these regions preferentially develop atherosclerosis when risk factors such as hypercholesterolemia are introduced.
Figures
Figure 1
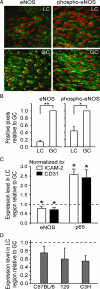
EC expression of eNOS protein and mRNA is reduced in an atherosclerosis-predisposed region of the normal mouse aorta. A: Representative immunoconfocal images of eNOS and phospho-eNOS (Ser1177) staining in the LC and GC of the ascending aorta from C57BL/6 mice fed standard laboratory chow. ECs in the LC display lower steady-state expression levels of eNOS and phospho-eNOS (green), compared with the GC. Nuclei were counterstained with propidium iodide (red). B: The extent of eNOS and phospho-eNOS staining in immunoconfocal images was quantified by assessing the percentage of positive pixels and comparing the LC with GC. These analyses confirm lower levels of eNOS and phospho-eNOS in the LC (*P < 0.05; **P < 0.01; n = 4). C: Real-time PCR quantification of eNOS and p65 mRNA levels in the LC intima relative to GC. Data were normalized to intercellular adhesion molecule-2 or CD31. The mRNA levels of eNOS were significantly lower in the LC region relative to GC (dashed line), whereas p65 mRNA levels were significantly higher (*P < 0.05; n = 7). D: Real-time PCR quantification of eNOS mRNA levels in LC and GC regions of three strains of mice: C57BL/6, 129, and C3H (n = 4).
Figure 2
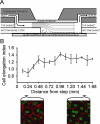
EC shape changes in response to disturbed laminar flow (DLF) and uniform laminar flow (ULF) in a parallel plate/step flow chamber. A: A schematic diagram of the chamber indicating regions of DLF and ULF. B: The shape of ECs along the flow path downstream of the step was quantified by assessing the elongation of nuclei. The index value is the mean ratio of nuclear dimensions parallel versus perpendicular to the direction of flow. A value of greater than 1 represents elongation in the direction of the flow. Representative confocal images of the DLF (left) and ULF (right) regions showing ECs immunostained with β-catenin, a protein localized at cell junctions. Nuclei were counterstained with propidium iodide.
Figure 3
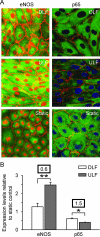
Expression of eNOS and p65 by porcine aortic ECs cultured in the DLF and ULF regions of the parallel-plate/step flow chamber, as well as under static conditions. A: Representative immunoconfocal images showing eNOS or p65 staining (green). Cell junctions were visualized by staining for β-catenin (red), and nuclei were counterstained either with propidium iodide (red) or TOTO-3 (blue). B: Quantification of eNOS and p65 expression (mean fluorescence intensities) in regions of DLF and ULF revealed opposing expression patterns. Data were standardized to expression levels in cells cultured under static conditions, which were assigned a value of 1. Boxed numbers above the bars represent the ratios of DLF to ULF. Significant differences are indicated (*P < 0.05; **P < 0.005; n = 4).
Figure 4
Effects of uniform laminar shear stress on the rates of eNOS and p65 transcription. A: Diagrams of the eNOS and p65 genes indicating the location of real-time PCR primers for hnRNA and mRNA. Black boxes represent exons and lines represent introns (FP, forward primer; RP, reverse primer). B: Alterations of eNOS and p65 hnRNA and mRNA expression levels in HAECs exposed to uniform laminar flow for 24 hours. Real-time PCR data were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) and compared with those in cells cultured under static conditions, which were assigned a value of 1. Significant differences are indicated (*P < 0.05; **P < 0.005; n = 4). C: RNA Pol II ChIP assays showing rates of eNOS and p65 transcription in HAECs exposed for 24 hours to uniform laminar flow (ULF) relative to cells cultured under static conditions. eNOS proximal promoter, p65 exon 1, and CD31 promoter DNA bound to RNA Pol II was immunoprecipitated and quantified by real-time PCR. Data for eNOS and p65 were normalized to CD31. Statistically significant differences between ULF and control values are indicated (*P < 0.05; n = 3).
Figure 5
Analysis of eNOS transcription and endogenous p65 protein expression in eNOS promoter-β-galactosidase reporter transgenic mice. Representative low (A) and high (B) magnification views of an ascending aorta histochemically stained for β-galactosidase, which is under the transcriptional control of the eNOS promoter. Diminished nuclear staining is seen in the LC. C: Representative confocal microscope images of the ascending aorta LC and GC regions immunostained for β-galactosidase (green). Nuclei were counterstained with propidium iodide (red). A markedly lower number of endothelial cell nuclei in the LC stain positively for β-galactosidase, reflecting a lower rate of eNOS transcription. D: Quantification of β-galactosidase expression in the GC and LC regions of the ascending aorta (*P < 0.001, n = 10). E: Immunoconfocal images showing inverse expression patterns of β-galactosidase (red) and endogenous p65 (green) in the LC and GC regions. The abundance of endogenous p65 protein is higher in the cytoplasm of LC endothelial cells, whereas the number of β-galactosidase-positive nuclei is lower. Nuclei were counterstained with TOTO-3 (blue). In C and D, the blood flow was from left to right.
Figure 6
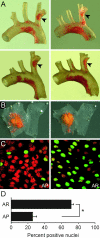
Mapping of atherosclerosis-prone (AP) and atherosclerosis-resistant (AR) regions in the brachiocephalic trunk (BCT) and analysis of eNOS transcription in these regions. A: Oil red O staining demonstrates reproducible atherosclerotic lesion formation at the origin of the right brachial artery from the BCT (arrowheads, posterior view) of four representative _ldlr_−/− mice fed a cholesterol-rich diet for 6 to 8 weeks. B: En face view of oil red O-stained BCT and proximal right brachial artery from two representative _ldlr_−/− mice fed a cholesterol-rich diet. The location of lesions was reproducible in five mice that were evaluated. C: Representative immunoconfocal images showing β-galactosidase staining in the AP and AR regions of the BCT in normocholesterolemic eNOS promoter-β-galactosidase reporter transgenic mice. D: Quantification of nuclei positive for β-galactosidase expression revealed a significant difference (*P < 0.05, n = 3) between the AP and AR regions.
Figure 7
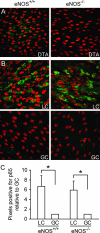
Expression of p65 by aortic endothelial cells in LC and GC regions of wild-type and eNOS−/− mice. A: Representative immunoconfocal images of the DTA from C57BL/6 wild-type (eNOS+/+) and eNOS−/− mice stained for eNOS (green) confirming the absence of eNOS expression in eNOS−/− mice. Nuclei were counterstained with propidium iodide (red). B: Representative immunoconfocal images of the LC and GC stained for p65 (green) showing similar topography of endogenous p65 protein expression in eNOS+/+ and eNOS−/− mice. Nuclei were counterstained with propidium iodide (red). C: Quantitative analysis of p65 staining in immunoconfocal images. The percentage of pixels positive for p65 staining was determined, and values of the GC and LC were compared (GC values were designated as 1). This analysis confirmed that the expression of p65 was comparable in eNOS+/+ and eNOS−/− mice. *P < 0.05.
Similar articles
- In Vivo Function of Flow-Responsive Cis-DNA Elements of eNOS Gene: A Role for Chromatin-Based Mechanisms.
Ku KH, Dubinsky MK, Sukumar AN, Subramaniam N, Feasson MYM, Nair R, Tran E, Steer BM, Knight BJ, Marsden PA. Ku KH, et al. Circulation. 2021 Aug 3;144(5):365-381. doi: 10.1161/CIRCULATIONAHA.120.051078. Epub 2021 Apr 29. Circulation. 2021. PMID: 33910388 - The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation.
Chan Y, Fish JE, D'Abreo C, Lin S, Robb GB, Teichert AM, Karantzoulis-Fegaras F, Keightley A, Steer BM, Marsden PA. Chan Y, et al. J Biol Chem. 2004 Aug 13;279(33):35087-100. doi: 10.1074/jbc.M405063200. Epub 2004 Jun 4. J Biol Chem. 2004. PMID: 15180995 - A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription.
Grumbach IM, Chen W, Mertens SA, Harrison DG. Grumbach IM, et al. J Mol Cell Cardiol. 2005 Oct;39(4):595-603. doi: 10.1016/j.yjmcc.2005.06.012. J Mol Cell Cardiol. 2005. PMID: 16099468 - Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel.
Tai SC, Robb GB, Marsden PA. Tai SC, et al. Arterioscler Thromb Vasc Biol. 2004 Mar;24(3):405-12. doi: 10.1161/01.ATV.0000109171.50229.33. Epub 2003 Dec 1. Arterioscler Thromb Vasc Biol. 2004. PMID: 14656742 Review. - Dysfunction of endothelial nitric oxide synthase and atherosclerosis.
Kawashima S, Yokoyama M. Kawashima S, et al. Arterioscler Thromb Vasc Biol. 2004 Jun;24(6):998-1005. doi: 10.1161/01.ATV.0000125114.88079.96. Epub 2004 Mar 4. Arterioscler Thromb Vasc Biol. 2004. PMID: 15001455 Review.
Cited by
- Gene regulation in the vascular endothelium: why epigenetics is important for the kidney.
Jamal A, Man HS, Marsden PA. Jamal A, et al. Semin Nephrol. 2012 Mar;32(2):176-84. doi: 10.1016/j.semnephrol.2012.02.009. Semin Nephrol. 2012. PMID: 22617766 Free PMC article. - Low Coronary Wall Shear Stress Is Associated With Severe Endothelial Dysfunction in Patients With Nonobstructive Coronary Artery Disease.
Kumar A, Hung OY, Piccinelli M, Eshtehardi P, Corban MT, Sternheim D, Yang B, Lefieux A, Molony DS, Thompson EW, Zeng W, Bouchi Y, Gupta S, Hosseini H, Raad M, Ko YA, Liu C, McDaniel MC, Gogas BD, Douglas JS, Quyyumi AA, Giddens DP, Veneziani A, Samady H. Kumar A, et al. JACC Cardiovasc Interv. 2018 Oct 22;11(20):2072-2080. doi: 10.1016/j.jcin.2018.07.004. Epub 2018 Sep 26. JACC Cardiovasc Interv. 2018. PMID: 30268874 Free PMC article. - Epigenetics in cardiovascular disease.
Shirodkar AV, Marsden PA. Shirodkar AV, et al. Curr Opin Cardiol. 2011 May;26(3):209-15. doi: 10.1097/HCO.0b013e328345986e. Curr Opin Cardiol. 2011. PMID: 21415727 Free PMC article. Review. - Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction.
Heo KS, Fujiwara K, Abe J. Heo KS, et al. Circ J. 2011;75(12):2722-30. doi: 10.1253/circj.cj-11-1124. Epub 2011 Nov 10. Circ J. 2011. PMID: 22076424 Free PMC article. Review. - Variant on 9p21 strongly associates with coronary heart disease, but lacks association with common stroke.
Lemmens R, Abboud S, Robberecht W, Vanhees L, Pandolfo M, Thijs V, Goris A. Lemmens R, et al. Eur J Hum Genet. 2009 Oct;17(10):1287-93. doi: 10.1038/ejhg.2009.42. Epub 2009 Mar 25. Eur J Hum Genet. 2009. PMID: 19319159 Free PMC article.
References
- Davies PF, Shi C, Depaola N, Helmke BP, Polacek DC. Hemodynamics and the focal origin of atherosclerosis: a spatial approach to endothelial structure, gene expression, and function. Ann NY Acad Sci. 2001;947:7–16. - PubMed
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis: quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. - PubMed
- Ku DN, Zhu C. The mechanical environment of the artery. Sumpio BE, editor. Austin, TX: R.G. Landes Company,; Hemodynamic Forces and Vascular Cell Biology. 1993:pp 1–23.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical