Integrin-dependent anchoring of a stem-cell niche - PubMed (original) (raw)
Integrin-dependent anchoring of a stem-cell niche
Guy Tanentzapf et al. Nat Cell Biol. 2007 Dec.
Abstract
Interactions between stem cells and their surrounding microenvironment, or niche, are critical for the establishment and maintenance of stem-cell properties. The adult Drosophila testis contains a morphologically discrete stem-cell niche, the 'hub'. The small cluster of non-dividing, somatic hub cells at the anterior tip of the fly testis is contacted by the germline stem cells (GSCs), which retain their stem-cell character through the direct association with the hub. Here we show that integrin-mediated adhesion is important for maintaining the correct position of embryonic hub cells during gonad morphogenesis. The misplaced hub in integrin-deficient embryos directs the orientation of cell divisions in the presumptive GSCs, a hallmark of the active germline stem-cell niche. A decrease in integrin-mediated adhesion in adult testes, which resulted in a loss of the hub and the stem-cell population, revealed the importance of hub-cell anchoring. Finally, we show that an extracellular matrix (ECM) is present around the gonad during late embryogenesis and that this ECM is defective in integrin-deficient gonads. On the basis of our data, we propose that integrins are required for the attachment of the hub cells to the ECM, which is essential for maintaining the stem-cell niche.
Figures
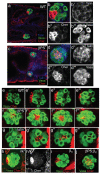
Figure 1. The hub cells are mispositioned in integrin mutants
(a, b) In wild-type stage 17 embryos the hub cells (labelled with Cheer, red) are positioned on the outer anterior edge of the male gonad (germline cells labeled with Vasa, green; Talin in blue labels the SGPs). (c, d) In mysXG43 embryos, which lack the βPS integrin subunit, the hub cells are partitioned to the middle of the testis, nestled in between the germline cells. (e-g) Confocal Z-series of 2 μm steps (labelled with Vasa (green) and Cheerio (red)) confirmed that, in contrast to wild-type embryos (e) where the hub cells were situated at the anterior pole of the gonad, the hub cells were found in the center of the gonad in embryos lacking integrins (f, mysXG43) or the integrin-associated protein talin (g, rhea79A). (h, h’) Hub cells were normally positioned in embryos that lack Integrin Linked Kinase (ilk1) and (i) in embryos that lack only the second integrin β subunit βν. (j) Gonad in embryo lacking both integrin β subunits (mysXG43; βν double mutant) had an identical phenotype to that seen in mysXG43 embryos. Scale bars 20 μm.
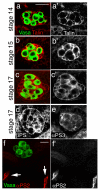
Figure 2. Integrin and talin expression in the somatic gonadal cells
(a-c) Talin protein (a’-c’, red in a-c with germline cells (Vasa) in green), encoded by the gene rhea, is expressed in all the SGPs starting at stage 14 (a) and persisting through stage 15 (b) to the end of embryogenesis (c, stage 17). Expression of integrins in the gonad: (d) It was previously reported that the integrin βPS subunit was not expressed in the gonad. We have now been able to detect its expression in all SGPs using new fixation methods but the staining is weak probably due to poor antibody penetration at late stages of embryogenesis. (e) The αPS3 subunit is also expressed in all SGPs. (f) The αPS2 subunit (f’, red in f with germline cells (Vasa) in green) is expressed in the muscle attachments (f, arrows) but is not detected in the gonad. Scale bars are 20 μm.
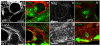
Figure 3. Integrins assemble an ECM around the embryonic gonad
(a, c) ECM proteins Ndg (a, a’, red) and LanA (c, c’, red) are localized around the embryonic gonad in wild-type embryos. The embryonic hub cells (labelled with Cher, green in a’ and c’) are in contact with the ECM. In integrin mutant embryos Ndg (b, b’, red) and LanA (d, d’, red) around the gonad was substantially reduced. Scale bars are 20 μm.
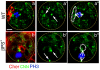
Figure 4. Spindle orientation in wild-type and integrin mutant embryonic gonads
(a) In mitotic GSCs of wild-type gonads (labelled with Phospho-Histone H3 specific antibody, blue) the centrosomes (labelled with CNN, green) are oriented so that the cell division is perpendicular to the anterior hub (labelled with Cheerio, red) and are generally aligned along the anterior-posterior axis of the embryo. (b) In integrin mutant gonads the centrosomes align perpendicular to the mispositioned hub. However, because the hub is the center of the gonad the plane of division is no longer strictly along the anterior-posterior axis. Scale bars are 10 μm.
Fig. 5. Phenotypes of talin RNAi in adult testes
UAS-Talin RNAi was expressed in embryonic and adult testis using Traffic Jam-Gal4. (a) In tj-GAL4/+; UAS-talin RNAi/+ (talin RNAi) embryos the hub cells localize to the anterior part of the gonad (Vasa, Green; Cher, red). (b) In a 2 week old adult tj-GAL4/tj-GAL4 testis the distal part of the testis holds the hub cells (b’, Cher, red), the GSCs, dividing gonial cells, and primary spermatocytes (b’, Vasa, green) and the rest of the testis fills with differentiated sperm bundles (arrow shows the distal tip of the testis). (c) A 2 week old adult talin RNAi testis contains only differentiated sperm, the hub cells (c’, Cher in red) are not at the distal testis tip (arrow), and no GSCs, gonial cells or primary spermatocytes are seen (c’, Vasa, green). (d, e) A magnified, close-up view of the distal tip of a 2 week old tj-GAL4 testis (d) and a talin RNAi testis (e). (d) In a tj-GAL4/tj-GAL4 testis Vasa (green) stains the GSCs, gonial cells and primary spermatocytes, no differentiated sperm bundles are observed. (e) In a talin RNAi testis the distal testis tip contains only differentiated sperm bundles (arrow in e) that extend to the apex of the testis (apex marked with an asterisk in d and e). (d’, e’) All somatic cells express talin in a _tj_-Gal4 testis (d’) but talin staining is absent in a talin RNAi testis (e’), though the gonadal sheath is stained. (d”, e”) In a tj-GAL4/tj-GAL4 testis (d”) the hub is located at the apex but in a talin RNAi testis (e”) the hub is missing (Vasa, green; Cher, red; and Talin, blue). (f-g) In a 3-day-old tj-GAL4tj-GAL4 testis (f) the tip of the testis contains the GSCs and germline cells at early stages of differentiation (arrow) but these are missing in a 3 day old talin RNAi. (h) An example of a 3-day-old talin RNAi testis where the hub is not localized to the distal tip (asterisk). (f-h; Vasa, green; Cher, red). Scale bars in b and c: 100μm, in a, d-h 20 μm.
Comment in
- Much HUBbub about stem-cell niches.
Van Doren M. Van Doren M. Nat Cell Biol. 2007 Dec;9(12):1344-5. doi: 10.1038/ncb1207-1344. Nat Cell Biol. 2007. PMID: 18059357
Similar articles
- Jak-STAT regulation of cyst stem cell development in the Drosophila testis.
Sinden D, Badgett M, Fry J, Jones T, Palmen R, Sheng X, Simmons A, Matunis E, Wawersik M. Sinden D, et al. Dev Biol. 2012 Dec 1;372(1):5-16. doi: 10.1016/j.ydbio.2012.09.009. Epub 2012 Sep 23. Dev Biol. 2012. PMID: 23010510 Free PMC article. - Maintenance of Stem Cell Niche Integrity by a Novel Activator of Integrin Signaling.
Lee JY, Chen JY, Shaw JL, Chang KT. Lee JY, et al. PLoS Genet. 2016 May 18;12(5):e1006043. doi: 10.1371/journal.pgen.1006043. eCollection 2016 May. PLoS Genet. 2016. PMID: 27191715 Free PMC article. - The receptor tyrosine phosphatase Lar regulates adhesion between Drosophila male germline stem cells and the niche.
Srinivasan S, Mahowald AP, Fuller MT. Srinivasan S, et al. Development. 2012 Apr;139(8):1381-90. doi: 10.1242/dev.070052. Epub 2012 Feb 29. Development. 2012. PMID: 22378638 Free PMC article. - The development of germline stem cells in Drosophila.
Dansereau DA, Lasko P. Dansereau DA, et al. Methods Mol Biol. 2008;450:3-26. doi: 10.1007/978-1-60327-214-8_1. Methods Mol Biol. 2008. PMID: 18370048 Free PMC article. Review. - Bam and Bgcn in Drosophila germline stem cell differentiation.
Perinthottathil S, Kim C. Perinthottathil S, et al. Vitam Horm. 2011;87:399-416. doi: 10.1016/B978-0-12-386015-6.00038-X. Vitam Horm. 2011. PMID: 22127253 Review.
Cited by
- The extracellular matrix: a dynamic niche in cancer progression.
Lu P, Weaver VM, Werb Z. Lu P, et al. J Cell Biol. 2012 Feb 20;196(4):395-406. doi: 10.1083/jcb.201102147. J Cell Biol. 2012. PMID: 22351925 Free PMC article. Review. - Visceral mesoderm signaling regulates assembly position and function of the Drosophila testis niche.
Anllo L, DiNardo S. Anllo L, et al. Dev Cell. 2022 Apr 25;57(8):1009-1023.e5. doi: 10.1016/j.devcel.2022.03.009. Epub 2022 Apr 6. Dev Cell. 2022. PMID: 35390292 Free PMC article. - Anchoring stem cells in the niche by cell adhesion molecules.
Xi R. Xi R. Cell Adh Migr. 2009 Oct-Dec;3(4):396-401. doi: 10.4161/cam.3.4.8604. Epub 2009 Oct 1. Cell Adh Migr. 2009. PMID: 19421010 Free PMC article. Review. - Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation.
König A, Yatsenko AS, Weiss M, Shcherbata HR. König A, et al. EMBO J. 2011 Apr 20;30(8):1549-62. doi: 10.1038/emboj.2011.73. Epub 2011 Mar 18. EMBO J. 2011. PMID: 21423150 Free PMC article. - Hybrid SMART spheroids to enhance stem cell therapy for CNS injuries.
Rathnam C, Yang L, Castro-Pedrido S, Luo J, Cai L, Lee KB. Rathnam C, et al. Sci Adv. 2021 Oct;7(40):eabj2281. doi: 10.1126/sciadv.abj2281. Epub 2021 Sep 29. Sci Adv. 2021. PMID: 34586845 Free PMC article.
References
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. - PubMed
- Xie T, Kawase E, Kirilly D, Wong MD. Intimate relationships with their neighbors: tales of stem cells in Drosophila reproductive systems. Dev Dyn. 2005;232:775–90. - PubMed
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. - PubMed
- Gilboa L, Lehmann R. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development. 2004;131:4895–905. - PubMed
- Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous