C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression - PubMed (original) (raw)
C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression
Michiko Hayashi et al. PLoS Genet. 2007 Nov.
Abstract
Chromosome inheritance during sexual reproduction relies on deliberate induction of double-strand DNA breaks (DSBs) and repair of a subset of these breaks as interhomolog crossovers (COs). Here we provide a direct demonstration, based on our analysis of rad-50 mutants, that the meiotic program in Caenorhabditis elegans involves both acquisition and loss of a specialized mode of double-strand break repair (DSBR). In premeiotic germ cells, RAD-50 is not required to load strand-exchange protein RAD-51 at sites of spontaneous or ionizing radiation (IR)-induced DSBs. A specialized meiotic DSBR mode is engaged at the onset of meiotic prophase, coincident with assembly of meiotic chromosome axis structures. This meiotic DSBR mode is characterized both by dependence on RAD-50 for rapid accumulation of RAD-51 at DSB sites and by competence for converting DSBs into interhomolog COs. At the mid-pachytene to late pachytene transition, germ cells undergo an abrupt release from the meiotic DSBR mode, characterized by reversion to RAD-50-independent loading of RAD-51 and loss of competence to convert DSBs into interhomolog COs. This transition in DSBR mode is dependent on MAP kinase-triggered prophase progression and coincides temporally with a major remodeling of chromosome architecture. We propose that at least two developmentally programmed switches in DSBR mode, likely conferred by changes in chromosome architecture, operate in the C. elegans germ line to allow formation of meiotic crossovers without jeopardizing genomic integrity. Our data further suggest that meiotic cohesin component REC-8 may play a role in limiting the activity of SPO-11 in generating meiotic DSBs and that RAD-50 may function in counteracting this inhibition.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
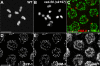
Figure 1. RAD-50 Is Required for Chiasma Formation but Not for Homolog Pairing and Synapsis
(A, B) Each panel shows the full karyotype in a single oocyte nucleus at diakinesis, the last stage of meiotic prophase. The wild-type nucleus (A) contains six DAPI-stained bodies, corresponding to the six pairs of homologous chromosomes (bivalents) attached by chiasmata, whereas the rad-50 mutant nucleus (B) contains 12 separate DAPI-stained chromosomes (univalents), indicating a lack of chismata connecting homolog pairs. (C) Pachytene nuclei from the rad-50 mutant stained with antibodies detecting X-chromosome pairing center-binding protein HIM-8 and chromosome axis protein HIM-3. A single HIM-8 focus (or two closely spaced HIM-8 foci) is seen in each nucleus, indicating successful homologous pairing. (D–F) Pachytene nuclei from the rad-50 mutant, showing coimmunolocalization of SC central region protein SYP-1 and meiotic chromosome axis protein HIM-3 at the interface between parallel tracks of DAPI-stained chromatin, indicating successful assembly of the SC. Scale bar = 5 μm.
Figure 2. Altered Profile of RAD-51 Foci in rad-50 Mutant Germ Lines
(A) Images show a portion of the germ lines of wild type and rad-50 mutant hermaphrodites, extending from the premeiotic region in which nuclei have not yet entered meiotic prophase (left) through the region of meiotic prophase entry (transition zone) and into the pachytene region (in which chromosomes are fully paired and synapsed; right). Chromosomes are stained with DAPI and RAD-51 antibody. In the wild-type germ line, RAD-51 foci are very infrequent in premeiotic and transition zone nuclei, but are abundant in pachytene nuclei, with multiple foci in the majority of nuclei. In the rad-50 mutant germ line, the number of foci in premeiotic and transition zone nuclei is elevated, while the number of foci in pachytene nuclei is severely reduced. Scale bar = 5 μm. (B) Quantitative time course analysis of RAD-51 foci. Stacked bar graph depicting quantitation of RAD-51 foci in premeiotic nuclei (pm), transition zone nuclei (tz), early-mid pachytene nuclei (e/m pt), and late pachytene nuclei (l pt) in gonads of the indicated genotypes. Different colored segments represent the percentage of nuclei scored that had the numbers of RAD-51 foci indicated by the color code at the right of the graph. (C) High magnification view of pachytene nuclei from wild type and spo-11; rad-50 germ lines. Whereas the RAD-51 foci in the wild-type pachytene nuclei are more numerous, the SPO-11-independent RAD-51 foci in the spo-11; rad-50 nuclei are larger/brighter. Scale bar = 5 μm.
Figure 3. Context-Specific Requirement for Rapid Accumulation of RAD-51 at Sites of IR-Induced Breaks
Germ lines from spo-11(ok79) and spo-11(ok79); rad-50 hermaphrodites fixed 1 h after exposure to 1 krad γ-irradiation and stained with DAPI and RAD-51 antibody. RAD-51 foci are detected in germ cell nuclei throughout the spo-11 gonad, from the proliferating nuclei at the distal tip (left) through nuclei at the diplotene stage of meiotic prophase (right). In the spo-11; rad-50 mutant, in contrast, RAD51 foci are absent in most nuclei in the central portion of the gonad, indicated by the bracket, from the onset of meiotic prophase through the mid-pachytene/late pachytene transition (see Figure 4). Scale bar = 5 μm.
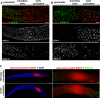
Figure 4. RAD-50 Dependence for Loading of RAD-51 at IR-Induced Breaks Extends from Meiotic Prophase Onset to the Mid-Pachytene/Late Pachytene Transition
(A, B) Images show a portion of the germ lines of rad-50 mutant hermaphrodites fixed 1 h after exposure to 1 krad γ-irradiation, centered on the transition zone and extending from prior to the onset of meiotic prophase (premeiotic, left) through early pachytene (right). (A) HTP-3 staining is diffusely associated with chromatin in premeiotic nuclei, then becomes concentrated on chromosome axis structures at the beginning of the transition zone, first appearing as discontinuous stretches and then as extended continuous linear structures. IR-induced RAD-51 foci are abundant in nuclei with diffuse HTP-3 staining, whereas nuclei with concentrated meiotic HTP-3 signals have very few RAD-51 foci. Scale bar = 5 μm (B) SYP-1 immunostaining is either absent or detected as a single bright focus in premeiotic nuclei, and is extensively localized on chromosomes beginning in the transition zone. IR-induced RAD-51 foci are abundant in nuclei lacking SYP-1, whereas nuclei with abundant SYP-1 have very few RAD-51 foci. Scale bar = 5 μm. (C) An unirradiated wild-type gonad and an irradiated rad-50 gonad stained with DAPI, α-RAD-51, and a monoclonal antibody detecting the activated, diphosphorylated form of MAP kinase (MAPK). Left, zoomed out view of the gonads (distal tip through diplotene), showing the position of the peak of MAP kinase activation that occurs at the mid-pachytene to late pachytene transition. Right, zoomed in view of the region surrounding this peak of activated MAP kinase, showing the location of this peak relative to RAD-51 foci. In the unirradiated wild-type germ line, RAD-51 foci peak in mid-pachytene and diminish in numbers prior to the peak of activated MAPK. In the irradiated rad-50 germ line, background levels of bright SPO-11-independent spontaneous RAD-51 foci are seen in a subset of nuclei throughout the gonad, whereas IR-induced RAD-51 foci abruptly rise in abundance in late pachytene nuclei, after the peak of activated MAPK. Left; Scale bar = 20 μm. Right; Scale bar = 5 μm.
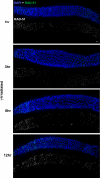
Figure 5. Time Course Analysis of IR-Induced RAD-51 Foci in the Constrained Region of spo-11; rad-50 Germ Lines
Images show portions of spo-11; rad-50 germ lines, centered on the constrained region and also including a subset of premeiotic nuclei (left) and late pachytene nuclei (right). Germ lines were fixed at the indicated times following exposure to 1 krad γ-irradiation and stained with DAPI and RAD-51 antibody. An obvious RAD-51-dark region in the central segment of the germ line is readily discernable at the 1, 3 and 6 h time points, but not at the 12 h time point. Scale bar = 5 μm.
Figure 6. RAD-50 Dependence for RAD-51 Loading Is Partially Abrogated in him-3 and htp-1 Mutants
Images show portions of germ lines extending from mid pachytene (left) through late pachytene/early diplotene (right) from hermaphrodites of the indicated genotypes that were exposed to 1 krad γ-irradiation 1 h prior to fixation. Both the rad-50 single mutant and the rad-50 syp-1 double mutant germ lines exhibit a “dark” region in which most nuclei lack RAD-51 foci, reflecting engagement of the RAD-50-dependent mode of RAD-51 loading that operates from meiotic prophase onset until the mid-pachytene to late pachytene transition. In the rad-50 syp-1 double mutant, the transition to nuclei with abundant IR-induced RAD-51 foci is observed at a more proximal position than in the rad-50 single mutant, at the very end of the pachytene stage, following exit from the persistent clustered chromosome configuration that is characteristic of mutants lacking SC central region components. In the him-3; rad-50 and htp-1; rad-50 double mutant germ lines shown, IR-induced RAD-51 foci are detected in nuclei throughout the mid-pachytene region, indicating that the requirement for RAD-50 is partially alleviated; however these IR-induced foci are less intense than those seen in the comparable regions of either the wild-type or htp-1 single mutant control germ lines. Scale bar = 5 μm.
Figure 7. Abnormal Chromosome Morphology Associated with rad-51 Mutants Is Suppressed in rad-51; rad-50 Double Mutants
Each panel shows the full karyotype in a single diakinesis-stage oocyte. Diakinesis oocytes in the rad-51 mutant typically have abnormal-appearing, poorly condensed chromosomes (left) and/or aggregated masses of chromosomes (middle), whereas diakinesis oocytes in the rad-51; rad-50 double mutant exhibit well-formed, non-aggregated univalent chromosomes (right). Scale bar = 2 μm.
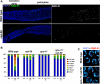
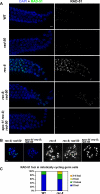
Figure 8. DSB Formation and RAD-51 Loading in the rec-8 Mutant Background
(A) Images show portions of the germ lines extending from mid-late pachytene through diplotene from worms of the indicated genotypes (these worms were NOT exposed to IR). Persistent high levels of meiotic RAD-51 foci are observed throughout this region in the rec-8 single mutant [full genotype: _rec-8(ok978) dpy-4(e1166)_]. In the rec-8; rad-50 double mutant [full genotype: _rec-8(ok978) dpy-4(e1166); rad-50(ok197)_], a region in which most nuclei lack RAD-51 foci (left) is followed by a region in which most nuclei have multiple RAD-51 foci. The transition between these states in the unirradiated rec-8; rad-50 gonad occurs at the very end of the pachytene region. This late pachytene rise in RAD-51 foci is absent in the spo-11 rec-8; rad-50 triple mutant [full genotype: _spo-11(me44) rec-8(ok978) dpy-4(e1166); rad-50(ok197)_], implying that these late foci are SPO-11-dependent. (B) Each panel shows the full karyotype in a single oocyte nucleus of the indicated genotype, either at the late diplotene/early diakinesis stage (left two panels, stained with DAPI [blue] and RAD-51 antibody [green]) or at late diakinesis (stained with DAPI in white; images shown are from the most mature oocyte in the gonad arm, in the −1 position immediately adjacent to the spermatheca). Multiple RAD-51 foci are detected in the late diplotene/early diakinesis nucleus from the rec-8; rad-50 worm, whereas the spo-11 rec-8; rad-50 nucleus at the same stage lacks RAD-51 foci. Similarly, multiple chromosome fragments are evident in the late diakinesis nucleus from the rec-8; rad-50 worm, whereas such fragments are not detected in the spo-11 rec-8; rad-50 late diakinesis nucleus (in which chromosomes are present largely as prematurely resolving pairs of sister chromatids). Scale bar = 2 μm. (C) Elevated levels of premeiotic RAD-51 foci in rec-8 mutant germ lines. RAD-51 foci were quantified for nuclei within the first 15 rows of germ cells, beginning at the distal tip of the gonad; three different germ lines were scored for each genotype (185 total nuclei for wild type, 177 total nuclei for rec-8). Stacked bar graph depicts the percentages of nuclei scored that had the indicated numbers of RAD-51 foci; the difference between rec-8 and wild type germ lines is extremely significant (p < 0.0001).
Similar articles
- BRC-1 acts in the inter-sister pathway of meiotic double-strand break repair.
Adamo A, Montemauri P, Silva N, Ward JD, Boulton SJ, La Volpe A. Adamo A, et al. EMBO Rep. 2008 Mar;9(3):287-92. doi: 10.1038/sj.embor.7401167. Epub 2008 Jan 25. EMBO Rep. 2008. PMID: 18219312 Free PMC article. - The C. elegans DSB-2 protein reveals a regulatory network that controls competence for meiotic DSB formation and promotes crossover assurance.
Rosu S, Zawadzki KA, Stamper EL, Libuda DE, Reese AL, Dernburg AF, Villeneuve AM. Rosu S, et al. PLoS Genet. 2013;9(8):e1003674. doi: 10.1371/journal.pgen.1003674. Epub 2013 Aug 8. PLoS Genet. 2013. PMID: 23950729 Free PMC article. - Disparate roles for C. elegans DNA translocase paralogs RAD-54.L and RAD-54.B in meiotic prophase germ cells.
Yamaya K, Wang B, Memar N, Odiba AS, Woglar A, Gartner A, Villeneuve AM. Yamaya K, et al. Nucleic Acids Res. 2023 Sep 22;51(17):9183-9202. doi: 10.1093/nar/gkad638. Nucleic Acids Res. 2023. PMID: 37548405 Free PMC article. - Meiotic recombination and the crossover assurance checkpoint in Caenorhabditis elegans.
Yu Z, Kim Y, Dernburg AF. Yu Z, et al. Semin Cell Dev Biol. 2016 Jun;54:106-16. doi: 10.1016/j.semcdb.2016.03.014. Epub 2016 Mar 21. Semin Cell Dev Biol. 2016. PMID: 27013114 Free PMC article. Review.
Cited by
- Meiotic Double-Strand Break Processing and Crossover Patterning Are Regulated in a Sex-Specific Manner by BRCA1-BARD1 in Caenorhabditis elegans.
Li Q, Hariri S, Engebrecht J. Li Q, et al. Genetics. 2020 Oct;216(2):359-379. doi: 10.1534/genetics.120.303292. Epub 2020 Aug 12. Genetics. 2020. PMID: 32796008 Free PMC article. - Where to Cross Over? Defining Crossover Sites in Plants.
Dluzewska J, Szymanska M, Ziolkowski PA. Dluzewska J, et al. Front Genet. 2018 Dec 12;9:609. doi: 10.3389/fgene.2018.00609. eCollection 2018. Front Genet. 2018. PMID: 30619450 Free PMC article. Review. - DNA repair, recombination, and damage signaling.
Gartner A, Engebrecht J. Gartner A, et al. Genetics. 2022 Feb 4;220(2):iyab178. doi: 10.1093/genetics/iyab178. Genetics. 2022. PMID: 35137093 Free PMC article. Review. - Fundamental cell cycle kinases collaborate to ensure timely destruction of the synaptonemal complex during meiosis.
Argunhan B, Leung WK, Afshar N, Terentyev Y, Subramanian VV, Murayama Y, Hochwagen A, Iwasaki H, Tsubouchi T, Tsubouchi H. Argunhan B, et al. EMBO J. 2017 Sep 1;36(17):2488-2509. doi: 10.15252/embj.201695895. Epub 2017 Jul 10. EMBO J. 2017. PMID: 28694245 Free PMC article. - A Distinct Class of Genome Rearrangements Driven by Heterologous Recombination.
León-Ortiz AM, Panier S, Sarek G, Vannier JB, Patel H, Campbell PJ, Boulton SJ. León-Ortiz AM, et al. Mol Cell. 2018 Jan 18;69(2):292-305.e6. doi: 10.1016/j.molcel.2017.12.014. Mol Cell. 2018. PMID: 29351848 Free PMC article.
References
- Page SL, Hawley RS. Chromosome choreography: the meiotic ballet. Science. 2003;301:785–789. - PubMed
- Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. - PubMed
- Colaiacovo MP, MacQueen AJ, Martinez-Perez E, McDonald K, Adamo A, et al. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell. 2003;5:463–474. - PubMed
- Assenmacher N, Hopfner KP. MRE11/RAD50/NBS1: complex activities. Chromosoma. 2004;113:157–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous