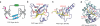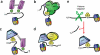How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers - PubMed (original) (raw)
Review
. 2007 Nov;14(11):1025-1040.
doi: 10.1038/nsmb1338. Epub 2007 Nov 5.
Affiliations
- PMID: 17984965
- PMCID: PMC4691843
- DOI: 10.1038/nsmb1338
Review
How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers
Sean D Taverna et al. Nat Struct Mol Biol. 2007 Nov.
Abstract
Histones comprise the major protein component of chromatin, the scaffold in which the eukaryotic genome is packaged, and are subject to many types of post-translational modifications (PTMs), especially on their flexible tails. These modifications may constitute a 'histone code' and could be used to manage epigenetic information that helps extend the genetic message beyond DNA sequences. This proposed code, read in part by histone PTM-binding 'effector' modules and their associated complexes, is predicted to define unique functional states of chromatin and/or regulate various chromatin-templated processes. A wealth of structural and functional data show how chromatin effector modules target their cognate covalent histone modifications. Here we summarize key features in molecular recognition of histone PTMs by a diverse family of 'reader pockets', highlighting specific readout mechanisms for individual marks, common themes and insights into the downstream functional consequences of the interactions. Changes in these interactions may have far-reaching implications for human biology and disease, notably cancer.
Figures
Figure 1
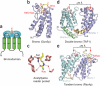
Histone post-translational modifications and their binding partners. (a) Stick models of different classes of post-translationally modified amino acid residuess, highlighting small chemical group side chains on histone tails. Yellow, carbon; blue, nitrogen; pink, polar hydrogen; red, oxygen; orange, phosphorus; green, methyl groups of post-translational modifications. Background is shaded by charge of side chains at physiological pH: light blue, positive; pink, negative; light green, uncharged. (b) Reading the histone (protein) code. Shown grouped by domain family are known chromatin-associated modules and the histone marks they have been reported to bind. Parentheses denote examples where structural information is known about related family members and their interactions with non-histone PTMs.
Figure 2
Readout of acetyllysine marks by bromodomains. (a) Topology of bromodomain fold. Green cylinders, core α-helices (αZ, αA, αB and αC); blue ovals, loops (LZA and LBC) that participate in acetyllysine reader pocket formation and also bear recognition elements for the modified peptide sequence; green triangle, approximate location of acetyllysine binding pocket. (b) Recognition of H4K16ac mark by the Gcn5p bromodomain (PDB 1E6I). Note insertion of the acetylated lysine side chain into a deep pocket generated at one end of the α-helix bundle. (c) Details of the Gcn5p acetyllysine binding pocket. Red spheres, water molecules; dashed lines, hydrogen bonds. For clarity, main chain portions of the cage residues and acetyllysine are omitted from stick models. (d,e) Examples of dual bromodomains. The TAF1 double bromodomain (d; PDB 1EQF) has been reported to function cooperatively in targeting properly separated diaceylated H4 tails. By contrast, the tandem bromodomain–containing Rsc4p (e; PDB 2R10) has been shown to recognize histone H3K14ac using its second bromodomain (pale cyan), whereas its first bromodomain (slate) is involved in recognition of an autoregulatory acetyllysine modification (K25ac) of the Rsc4p protein itself. Slate and pale cyan, N- and C-terminal bromodomains, respectively; red arrows, acetyllysine reader pockets; measuring bar, approximate distance separating the two pockets. In b, d, e and subsequent figures, all reader modules are depicted as ribbons, with key cage side chains shown as pink sticks; histone peptides bearing acetyllysine or other types of PTMs are in yellow, and modified residues are colored as in Figure 1a; N and C termini of the effector proteins are marked in black and those of histone peptides in red.
Figure 3
Readout of methyllysine marks by Royal-superfamily modules. (a) Topology of the Royal-superfamily fold. Blue arrows, β-strands that form an incomplete β-barrel reminiscent of the SH3 domain fold; orange ovals, loops participating in methyllysine reader pocket formation; light blue circle, binding pocket at one end of the module. Classical chromo modules have only three core β-strands (labeled 2–4) and one orphaned extra strand (labeled 5). Upon complex formation, the histone peptide completes this five-stranded β-barrel fold by introducing an extra β-strand at position 1 prime, sandwiched between strands 2 and 5 (see b). This sandwiching binding mode occurs mainly with chromodomains. In other Royal superfamily members, the interactions are more varied; however, docking in the β-strand conformation to extend one edge of an existing β-sheet is not uncommon (see d). (b–f) Examples of known complex structures in the Royal superfamily, ranging from higher methylation state–specific readers (b–d) to lower methylation state–specific readers (e and f). Strands that form the SH3-like β-barrel are in slate, numbered as in a. Coordinates have PDB codes 1KNE (b), 2B2W (c), 2GFA (d), 2IG0 (e) and 2PQW (f).
Figure 4
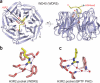
Readout of modified and unmodified histone lysine marks by PHD finger modules. (a) Topology of the PHD finger fold. Blue arrows, two small β-strands that bridge the interleaved zinc-finger motifs; labeled white circles, zinc-coordinated cysteine and histidine residues; green cylinder, short α-helix (α1) near the C terminus; pink circles, the caging residues for readout of methyllysine or unmodified lysine marks. (b) Specific recognition of the H3K4me3 mark by BPTF PHD finger (PDB 2F6J). The H3 peptide resides in a surface groove between α1 and β-strand 1 of BPTF PHD finger upon formation of the antiparallel β-sheet with the core β-strands. Note the full aromatic cage formed at the protein surface for trimethyl-specific readout. H3K4 site specificity is achieved by simultaneous recognition of H3K4me3 and H3R2, as well as by anchoring of the N terminus by the BPTF PHD finger. Similar strategies for readout of H3K4me3 marks have been observed for ING2 and Yng1p PHD fingers. Cyan spheres, zinc ions within the zinc-finger motifs. (c,d) Specific readout of unmodified H3K4 peptide by BHC80 PHD finger (c; PDB 2PUY) and DNMT3L ADD domain (a PHD finger–containing module; d; 2PVC). Both modules have a surface patch of acidic residues for unmodified H3K4 mark recognition. Similar β-sheet–formation and flanking residue–recognition strategies have been observed for site-specific readout of H3 peptides. In d, the PHD finger module (slate) is embedded within the cysteine-rich ADD domain, which contains an additional zinc-finger motif.
Figure 5
Readout of an unmodified arginine by the WD40 repeat of WDR5. (a) Top view (left) and side view (right) of WDR5 in complex with H3K4me2 peptide (PDB 2H6N). H3R2 and H3K4me2 are in stick representation. H3R2 is deeply buried in the central cavity of WDR5, whereas H3K4me2 is presented by WDR5 on the protein surface for further methylation. (b,c) Details of H3R2 readout by WDR5 in a cavity-insertion recognition mode (b) and by BPTF PHD finger in a surface-groove recognition mode (c).
Figure 6
Readout of phosphoserine marks by 14-3-3 and BRCT domains. (a) Overview of the complex structure of 14-3-3ζ bound to H3S10ph peptide (PDB 2C1J). Peptide is buried in the V-shaped 14-3-3 protein scaffold. The H3K9ac mark can be accommodated on the surface channel of 14-3-3. (b) Details of H3S10ph mark recognition around the phosphoserine-binding site within 14-3-3ζ. The phosphate group is anchored by multiple hydrogen-bonding and ion-pair networks. (c) Details of H3R8 recognition by 14-3-3ζ. Recognition of the guanidinium amino group of H3R8 contributes to sequence specificity of H3S10ph readout by 14-3-3ζ. The intramolecular contact to the phosphate in the bound peptide is stabilized by additional contacts involving each residue. Similar contacts are anticipated in phosphohistone complexes with other 14-3-3 isoforms. (d) Overview of structure of the MDC1 tandem BRCT domains in complex with a H2AXS139ph peptide (PDB 2AZM). Slate and pale cyan, N- and C-terminal BRCT domains, respectively; beige, helix-loop-helix linker. The peptide-binding site lies at the interface of the two domains. (e) Close-up view of the phosphoserine-binding site. The site is formed within the first BRCT domain and stabilized by several hydrogen bonds with pronounced coulombic character, involving side chains as well as main chains of several BRCT domain residues. (f) Close-up view of recognition of the C-terminal tyrosine (+3 position) at the interface of the two MDC1 BRCA domains. The free C-terminal carboxylate group is capped by an arginine side chain from the first BRCT domain. The tyrosine side chain is stacked against a proline ring from the second BRCT domain. This provides another example of terminus specificity as an apparent additional recognition determinant.
Figure 7
Combinations of PTM-binding sites generate different specifities. (a) The double bromodomain motif in hTAF1 simultaneously binds proximal acetylated lysines on H3 or H4. (b) First and second modules of L3MBTL1 may simultaneously bind proline and dimethyllysine marks on histone tails. (c) The PHD finger of BPTF and the proximal bromodomain bind H3K4me3 and an acetyllysine simultaneously. (d) The PHD finger of Rco1p increases the binding of the Eaf3p chromobarrel to H3K36me3 nucleosomes. (e) Schematic of histone-directed protease activity uncovering a cryptic PTM-binding site.
Similar articles
- Cooperative binding of two acetylation marks on a histone tail by a single bromodomain.
Morinière J, Rousseaux S, Steuerwald U, Soler-López M, Curtet S, Vitte AL, Govin J, Gaucher J, Sadoul K, Hart DJ, Krijgsveld J, Khochbin S, Müller CW, Petosa C. Morinière J, et al. Nature. 2009 Oct 1;461(7264):664-8. doi: 10.1038/nature08397. Nature. 2009. PMID: 19794495 - TATA-binding protein-free TAF-containing complex (TFTC) and p300 are both required for efficient transcriptional activation.
Hardy S, Brand M, Mittler G, Yanagisawa J, Kato S, Meisterernst M, Tora L. Hardy S, et al. J Biol Chem. 2002 Sep 6;277(36):32875-82. doi: 10.1074/jbc.M205860200. Epub 2002 Jul 9. J Biol Chem. 2002. PMID: 12107188 - MYC interacts with the human STAGA coactivator complex via multivalent contacts with the GCN5 and TRRAP subunits.
Zhang N, Ichikawa W, Faiola F, Lo SY, Liu X, Martinez E. Zhang N, et al. Biochim Biophys Acta. 2014 May;1839(5):395-405. doi: 10.1016/j.bbagrm.2014.03.017. Epub 2014 Apr 3. Biochim Biophys Acta. 2014. PMID: 24705139 Free PMC article. - Interpreting the language of histone and DNA modifications.
Rothbart SB, Strahl BD. Rothbart SB, et al. Biochim Biophys Acta. 2014 Aug;1839(8):627-43. doi: 10.1016/j.bbagrm.2014.03.001. Epub 2014 Mar 12. Biochim Biophys Acta. 2014. PMID: 24631868 Free PMC article. Review. - Readout of epigenetic modifications.
Patel DJ, Wang Z. Patel DJ, et al. Annu Rev Biochem. 2013;82:81-118. doi: 10.1146/annurev-biochem-072711-165700. Annu Rev Biochem. 2013. PMID: 23642229 Free PMC article. Review.
Cited by
- Targeting of TAMs: can we be more clever than cancer cells?
Kzhyshkowska J, Shen J, Larionova I. Kzhyshkowska J, et al. Cell Mol Immunol. 2024 Nov 8. doi: 10.1038/s41423-024-01232-z. Online ahead of print. Cell Mol Immunol. 2024. PMID: 39516356 Review. - Tracing the Chromatin: From 3C to Live-Cell Imaging.
Lacen AN, Lee HT. Lacen AN, et al. Chem Biomed Imaging. 2024 Jun 25;2(10):659-682. doi: 10.1021/cbmi.4c00033. eCollection 2024 Oct 28. Chem Biomed Imaging. 2024. PMID: 39483638 Free PMC article. Review. - Histone demethylase KDM2A recruits HCFC1 and E2F1 to orchestrate male germ cell meiotic entry and progression.
Feng S, Gui Y, Yin S, Xiong X, Liu K, Li J, Dong J, Ma X, Zhou S, Zhang B, Yang S, Wang F, Wang X, Jiang X, Yuan S. Feng S, et al. EMBO J. 2024 Oct;43(19):4197-4227. doi: 10.1038/s44318-024-00203-4. Epub 2024 Aug 19. EMBO J. 2024. PMID: 39160277 Free PMC article. - Tracking live-cell single-molecule dynamics enables measurements of heterochromatin-associated protein-protein interactions.
Chen Z, Seman M, Fyodorova Y, Farhat A, Ames A, Levashkevich A, Biswas S, Huang F, Freddolino L, Biteen JS, Ragunathan K. Chen Z, et al. Nucleic Acids Res. 2024 Oct 14;52(18):10731-10746. doi: 10.1093/nar/gkae692. Nucleic Acids Res. 2024. PMID: 39142658 Free PMC article. - Using dimethylsulfonium to identify readers of methylation.
Li X, Li XD. Li X, et al. Nat Chem. 2024 Aug;16(8):1221-1222. doi: 10.1038/s41557-024-01582-1. Nat Chem. 2024. PMID: 39079946 No abstract available.
References
- Allis CD, Jenuwein T, Reinberg D, Caparros ML, editors. Epigenetics. Cold Spring Harbor Laboratory Press; Woodbury, New York: 2006.
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. - PubMed
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. - PubMed
- Pokholok DK, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. - PubMed
- Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 GM063959/GM/NIGMS NIH HHS/United States
- GM63959/GM/NIGMS NIH HHS/United States
- R37 GM053512/GM/NIGMS NIH HHS/United States
- GM53512/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous