Cross-presentation of NY-ESO-1 cytotoxic T lymphocyte epitope fused to human heat shock cognate protein 70 by dendritic cells - PubMed (original) (raw)
Cross-presentation of NY-ESO-1 cytotoxic T lymphocyte epitope fused to human heat shock cognate protein 70 by dendritic cells
Seiya Susumu et al. Cancer Sci. 2008 Jan.
Abstract
The cancer-testis antigen NY-ESO-1 has been implicated as one of the most attractive candidates for a cancer vaccine. However, a protein vaccine generally meets inefficient antigen presentation to CD8(+) T cells, which could be overcome by combination with an appropriate adjuvant. Heat shock protein is a natural adjuvant and activates the antigen-presenting cells to channel exogenous antigens into the classical major histocompatibility complex class I antigen-processing pathway (cross-presentation). Therefore, we genetically fused a minigene encompassing the NY-ESO-1 cytotoxic T lymphocyte (CTL) epitope 157-165 (ESO p157-165) to the human heat shock cognate protein 70 (hsc70) and expressed the resulting fusion proteins in Escherichia coli. By using a human leukocyte antigen-A*0201-restricted NY-ESO-1-specific CTL clone, the cross-presentation of ESO p157-165 by monocyte-derived dendritic cells (mo-DC) pulsed with the fusion protein was evaluated. The fusion protein-pulsed mo-DC activates the CTL clone much more efficiently than the free NY-ESO-1 protein-pulsed mo-DC. Moreover, the magnitude of the CTL activity was comparable between ESO p157-165 and the fusion protein of hsc70 and ESO p157-165 (hsc70-ESO p157-165 fusion protein). In addition, the CTL activation induced by the fusion protein, but not by the epitope, was inhibited by paraformaldehyde fixation of the mo-DC and by treatment with lactacystin, a specific inhibitor for the proteasome. Finally, the hsc70-ESO p157-165 fusion protein-pulsed DC was able to induce an antigen-specific T-cell response. These results suggest that the hsc70-ESO p157-165 fusion protein is therefore considered to be a promising candidate as a cancer vaccine.
Figures
Figure 1
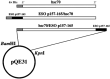
Construction of plasmids encoding human heat shock cognate protein 70 (hsc70) and NY‐ESO‐1 cytotoxic T lymphocyte (CTL) epitope fusion genes. A minigene encoding the NY‐ESO‐1 CTL epitope 157‐165 was fused to the N‐ or C‐terminus of the hsc70 gene. These fusion genes were inserted into the pQE31 expression vector via 5′_Bam_HI and 3′_Kpn_I restriction sites.
Figure 2
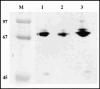
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) of the fusion proteins. NY‐ESO‐1 cytotoxic T lymphocyte epitope 157‐165 (ESO p157‐165) was conjugated with human heat shock cognate protein 70 (hsc70) at either the C‐ or N‐terminus. Both of these fusion proteins were detected on the Coomassie‐stained SDS‐PAGE. Lane 1, hsc70–ESO p157‐165; lane 2, ESO p157‐165/hsc70; lane 3, hsc70; M, marker.
Figure 3
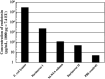
Removal of endotoxin from lysates of Escherichia coli. After purification, these fusion proteins were treated with Kurimover I and II to remove contaminated lipopolysaccharide (LPS). The concentration of LPS, determined by the Limulus ES‐II test, was less than 50 pg/mL (less than 2.0 endotoxin unit [EU]/mg fusion proteins).
Figure 4
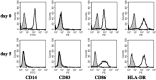
Phenotype of monocytes and monocyte‐derived dendritic cells. The monocytes isolated by magnetic‐bead selection from the peripheral blood mononuclear cells were harvested immediately (day 0) and cultured with granulocyte/macrophage colony‐stimulating factor (GM‐CSF) and interleukin (IL)‐4 for 5 days. The monocyte‐derived dendritic cells were used in the following assays.
Figure 5
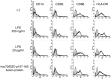
Maturation of monocyte‐derived dendritic cells (mo‐DC). On day 6, mo‐DC were stimulated with lipopolysaccharide (LPS) (250 ng/mL or 20 pg/mL at final concentration) or the fusion protein of human heat shock cognate protein 70 (hsc70) and ESO p157‐165 (hsc70–ESO p157‐165 fusion protein) (20 pg/mL LPS and 350 µg/mL protein at final concentration), and the cells were harvested 24 h later. The cells were stained using the antibodies indicated, and were analyzed by flow cytometry.
Figure 6
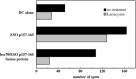
Presentation of NY‐ESO‐1 cytotoxic T lymphocyte (CTL) epitope to NY‐ESO‐1‐specific CD8+ T cells by monocyte‐derived dendritic cells (mo‐DC) exposed to the fusion protein of human heat shock cognate protein 70 (hsc70) and ESO p157‐165 (hsc70–ESO p157‐165 fusion protein). The mo‐DC from a human leukocyte antigen (HLA)‐A*0201‐positive healthy donor were exposed for 2 h in plain RPMI with a NY‐ESO‐1 synthetic peptide (ESO p157‐165 at 5 µg/mL), hsc70–ESO p157‐165 fusion protein (at 350 µg/mL), recombinant hsc70 (at 350 µg/mL), or NY‐ESO‐1 protein (at 16 µg/mL). The two clones, namely clones 5 and 49, from a single donor were used as HLA‐A2‐restricted NY‐ESO‐1‐specific CD8+ T‐cell clones. (a) A photograph of the wells in an enzyme‐linked immunospot assay using clone 5. (b) The data in the panel represent the average number of positive spots stimulated by clone 49.
Figure 7
Paraformaldehyde inhibits cross‐presentation of a NY‐ESO‐1 cytotoxic T lymphocyte (CTL) epitope. The monocyte‐derived dendritic cells (mo‐DC) were harvested at day 5, then fixed for 15 min with 1% paraformaldehyde in phosphate‐buffered saline (pH 7.4), and used for assays. The enzyme‐linked immunospot assays using the human leukocyte antigen‐A2‐restricted NY‐ESO‐1‐specific CD8+ T‐cell clone were carried out as described above. Solid bars, no paraformaldehyde; gray bars, paraformaldehyde added.
Figure 8
Lactacystin inhibits cross‐presentation of a NY‐ESO‐1 cytotoxic T lymphocyte (CTL) epitope. The monocyte‐derived dendritic cells (mo‐DC) were harvested at day 5, then cultured for an additional 12 h in the presence or absence of lactacystin (10 µM) in normal growth medium, and used for assays. The enzyme‐linked immunospot assays using the human leukocyte antigen‐A2‐restricted NY‐ESO‐1‐specific CD8+ T‐cell clone were carried out as described above. Solid bars, no lactacystin; gray bars, lactacystin added.
Figure 9
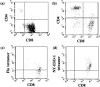
ESO p157‐165‐specific T cells detected by tetramer. (a) The CD8+ cells were isolated using magnetic beads. (b) After three stimulations by monocyte‐derived dendritic cells (mo‐DC) pulsed with human heat shock cognate protein 70–ESO p157‐165 fusion protein, CD4+ T cells whose antigen specificity was unknown grew. The expanded CD8+ T cells were detected specifically by human leukocyte antigen‐A2 tetramer complexed with ESO p157‐165 (d) but not with influenza A matrix p58‐66 (c).
Similar articles
- Efficient induction of a Her2-specific anti-tumor response by dendritic cells pulsed with a Hsp70L1-Her2(341-456) fusion protein.
Fu Q, Wu Y, Yan F, Wang N, Wang W, Cao X, Wang Y, Wan T. Fu Q, et al. Cell Mol Immunol. 2011 Sep;8(5):424-32. doi: 10.1038/cmi.2011.21. Epub 2011 Jul 25. Cell Mol Immunol. 2011. PMID: 21785448 Free PMC article. - Processing and cross-presentation of individual HLA-A, -B, or -C epitopes from NY-ESO-1 or an HLA-A epitope for Melan-A differ according to the mode of antigen delivery.
Robson NC, McAlpine T, Knights AJ, Schnurr M, Shin A, Chen W, Maraskovsky E, Cebon J. Robson NC, et al. Blood. 2010 Jul 15;116(2):218-25. doi: 10.1182/blood-2009-10-249458. Epub 2010 Apr 29. Blood. 2010. PMID: 20430956 - Hsp70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine.
Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N, Chen G, Dai S, Liu S, Zhang M, Cao X. Wu Y, et al. Cancer Res. 2005 Jun 1;65(11):4947-54. doi: 10.1158/0008-5472.CAN-04-3912. Cancer Res. 2005. PMID: 15930317 - Oral vaccination with attenuated Salmonella enterica strains encoding T-cell epitopes from tumor antigen NY-ESO-1 induces specific cytotoxic T-lymphocyte responses.
Meng JZ, Dong YJ, Huang H, Li S, Zhong Y, Liu SL, Wang YD. Meng JZ, et al. Clin Vaccine Immunol. 2010 Jun;17(6):889-94. doi: 10.1128/CVI.00044-10. Epub 2010 Apr 7. Clin Vaccine Immunol. 2010. PMID: 20375244 Free PMC article. - Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL.
Chen JL, Dunbar PR, Gileadi U, Jäger E, Gnjatic S, Nagata Y, Stockert E, Panicali DL, Chen YT, Knuth A, Old LJ, Cerundolo V. Chen JL, et al. J Immunol. 2000 Jul 15;165(2):948-55. doi: 10.4049/jimmunol.165.2.948. J Immunol. 2000. PMID: 10878370
Cited by
- Antitumor immune responses mediated by dendritic cells: How signals derived from dying cancer cells drive antigen cross-presentation.
Spel L, Boelens JJ, Nierkens S, Boes M. Spel L, et al. Oncoimmunology. 2013 Nov 1;2(11):e26403. doi: 10.4161/onci.26403. Epub 2013 Oct 21. Oncoimmunology. 2013. PMID: 24482744 Free PMC article. Review. - An engineered non-toxic superantigen increases cross presentation of hepatitis B virus nucleocapsids by human dendritic cells.
McIntosh JD, Manning K, Chokshi S, Naoumov NV, Fraser JD, Dunbar PR, Taylor JA. McIntosh JD, et al. PLoS One. 2014 Apr 1;9(4):e93598. doi: 10.1371/journal.pone.0093598. eCollection 2014. PLoS One. 2014. PMID: 24690680 Free PMC article. - Both CD4+ and CD8+ T cell epitopes fused to heat shock cognate protein 70 (hsc70) can function to eradicate tumors.
Mizukami S, Kajiwara C, Ishikawa H, Katayama I, Yui K, Udono H. Mizukami S, et al. Cancer Sci. 2008 May;99(5):1008-15. doi: 10.1111/j.1349-7006.2008.00788.x. Epub 2008 Mar 12. Cancer Sci. 2008. PMID: 18341654 Free PMC article. - Acid-degradable polyurethane particles for protein-based vaccines: biological evaluation and in vitro analysis of particle degradation products.
Bachelder EM, Beaudette TT, Broaders KE, Paramonov SE, Dashe J, Fréchet JM. Bachelder EM, et al. Mol Pharm. 2008 Sep-Oct;5(5):876-84. doi: 10.1021/mp800068x. Epub 2008 Aug 19. Mol Pharm. 2008. PMID: 18710254 Free PMC article. - Immunological milieu in the peritoneal cavity at laparotomy for gastric cancer.
Yoneda A, Ito S, Susumu S, Matsuo M, Taniguchi K, Tajima Y, Eguchi S, Kanematsu T, Nagata Y. Yoneda A, et al. World J Gastroenterol. 2012 Apr 7;18(13):1470-8. doi: 10.3748/wjg.v18.i13.1470. World J Gastroenterol. 2012. PMID: 22509078 Free PMC article.
References
- Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun 2004; 4: 1. - PubMed
- Van Der Brüggen P, Zhang Y, Chaux P et al . Tumor‐specific shared antigenic peptides recognized by human T cells. Immunol Rev 2002; 188: 51–64. - PubMed
- Jäger E, Jäger D, Knuth A. Antigen‐specific immunotherapy and cancer vaccines. Int J Cancer 2003; 106: 817–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous