DNA methylation inhibitor 5-Aza-2'-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B - PubMed (original) (raw)
DNA methylation inhibitor 5-Aza-2'-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B
Stela S Palii et al. Mol Cell Biol. 2008 Jan.
Abstract
Genome-wide DNA methylation patterns are frequently deregulated in cancer. There is considerable interest in targeting the methylation machinery in tumor cells using nucleoside analogs of cytosine, such as 5-aza-2'-deoxycytidine (5-azadC). 5-azadC exerts its antitumor effects by reactivation of aberrantly hypermethylated growth regulatory genes and cytoxicity resulting from DNA damage. We sought to better characterize the DNA damage response of tumor cells to 5-azadC and the role of DNA methyltransferases 1 and 3B (DNMT1 and DNMT3B, respectively) in modulating this process. We demonstrate that 5-azadC treatment results in growth inhibition and G(2) arrest-hallmarks of a DNA damage response. 5-azadC treatment led to formation of DNA double-strand breaks, as monitored by formation of gamma-H2AX foci and comet assay, in an ATM (ataxia-telangiectasia mutated)-dependent manner, and this damage was repaired following drug removal. Further analysis revealed activation of key strand break repair proteins including ATM, ATR (ATM-Rad3-related), checkpoint kinase 1 (CHK1), BRCA1, NBS1, and RAD51 by Western blotting and immunofluorescence. Significantly, DNMT1-deficient cells demonstrated profound defects in these responses, including complete lack of gamma-H2AX induction and blunted p53 and CHK1 activation, while DNMT3B-deficient cells generally showed mild defects. We identified a novel interaction between DNMT1 and checkpoint kinase CHK1 and showed that the defective damage response in DNMT1-deficient cells is at least in part due to altered CHK1 subcellular localization. This study therefore greatly enhances our understanding of the mechanisms underlying 5-azadC cytotoxicity and reveals novel functions for DNMT1 as a component of the cellular response to DNA damage, which may help optimize patient responses to this agent in the future.
Figures
FIG. 1.
Responses of HeLa and HCT116 cells to the DNA methylation inhibitor 5-azadC and the role of DNMT1 and DNMT3B. (A) Effect of increasing concentrations of 5-azadC on DNMT1, DNMT3A, and DNMT3B protein levels. HeLa cells were mock treated (−) or treated with 0.1, 0.5, 1.0, 5.0, and 10.0 μM 5-azadC for 48 h (indicated by the wedge); then soluble protein extracts were prepared and subjected to SDS-PAGE followed by Western blotting with the antibodies indicated at the left. GAPDH served as a loading control. (B) Effect of 5-azadC on cell viability. Cells were treated with indicted doses of 5-azadC for 48 h (fresh drug added every 24 h); the medium was changed, and then cell viability was determined using the MTT assay. (C) 5-azadC treatment results in decreased clonogenic survival. Cell lines were treated with 5-azadC for 48 h, followed by replacement of the medium and continued growth for 10 to 12 days. Results are presented as the average of quadruplicate measurements, and the bar is the standard deviation. (D) Effect of increasing doses of 5-azadC on the cell cycle summarized as the relative increase in the number of cells (_n_-fold) arrested in G2/M for each of the cell lines following drug treatment. Experiments were repeated three times and averaged. (E) HeLa cells treated with 10 μM 5-azadC for 48 h preferentially arrest in G2 as determined by DNA content (PI staining shown on the x axis) and staining cells with an antibody for the M phase specific marker phospho-Ser10 histone H3 (y axis) followed by flow cytometry.
FIG. 2.
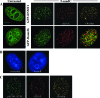
Treatment of cells with 5-azadC results in induction of γ-H2AX, a marker of DNA double-strand breaks, that is dependent on DNMT1 and the PI3K ATM. (A) Representative immunofluorescence staining result for γ-H2AX (red) and DNA (blue) in untreated and 10 μM 5-azadC-treated HCT116 cells. A magnified view of the boxed cell is shown in the upper left corner of the 5-azadC-treated cells. (B) Dose-dependent increase in γ-H2AX-positive HeLa, HCT116 parental, 1KO, and 3BKO cells treated with 5-azadC (left graph). Induction of γ-H2AX staining in isogenic YZ5 and EBS cell lines following 5-azadC treatment (right graph). Results are plotted as the average relative change in cells showing five or more γ-H2AX foci compared to untreated cells. Data are graphed as the average increase (_n_-fold) in level of staining, relative to the respective parental cell lines (set at 1.0). (C) Quantitation of the percentage of γ-H2AX-positive cells and level of apoptosis (inset graph) in each cell line in the absence of any drug treatment (basal levels) showing that cell lines with reduced or absent induction of γ-H2AX staining upon 5-azadC treatment (1KO and EBS) exhibit elevated basal levels of γ-H2AX expression and apoptosis. (D) PI3Ks are required for H2AX phosphorylation in response to 5-azadC treatment. HeLa and parental HCT116 cells were mock treated (UT), treated with 10 μM 5-azadC alone for 24 h (5-azadC), or treated with both 10 μM 5-azadC and 10 μM wortmannin (a general PI3K inhibitor) for 24 h (A+W); then, cells were fixed and stained with a γ-H2AX antibody, and positive cells were quantitated (cells with five or more γ-H2AX foci). All values are an average of at least three experiments, and the error bar is standard deviation from the mean.
FIG. 3.
5-azadC treatment results in formation of DNA strand breaks that are repaired upon drug withdrawal. (A) Experimental scheme used for the assays in this figure. Cells were treated with 5-azadC for 48 h with fresh drug added each day. The medium was then changed, and the cells were allowed to recover for 4 days. Numbering in all graphs is relative to the day post-drug withdrawal, which starts at zero hours. (B) Growth inhibition of all cell lines after removal of 5-azadC. Results are presented as the average percent growth inhibition ([100 − (number of cells in treated sample/number of cells in untreated sample)] × 100) for quadruplicate experiments. (C) Soluble DNMT protein levels before and during drug treatment and recovery as monitored by Western blotting. Ku70 served as a loading control. Hrs, hours. (D) Time course (or recovery) of γ-H2AX staining in HeLa cells following withdrawal of 5-azadC (left). Time course of HCT116 parental, 1KO, and 3BKO cells following 5-azadC withdrawal from the growth medium (right). (E) Representative comet assay result showing formation of DNA strand breaks (formation of a “comet tail”) in 5-azadC-treated HCT116 cells. The photograph is taken at a magnification of ×20. (F) Time course of comet tail formation in HeLa cells (left) or HCT116, 1KO, and 3BKO cells (right) following withdrawal of 5-azadC from the medium. Results are presented as the relative change (_n_-fold) in cells displaying a comet tail compared to untreated cells. Experiments were repeated at least twice, and the results shown are representative.
FIG. 4.
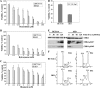
DNA damage response to 5-azadC, as monitored by quantitative Western blotting, and the role of DNMT1 and DNMT3B. (A) The HCT116 cell lines indicated at the bottom of the Western panel were treated with 10 μM 5-azadC for 0, 24, 48, and 72 h (with fresh drug added every 24 h), and whole-cell extracts were prepared. Equal microgram amounts of extract were loaded onto SDS-PAGE gels, transferred to polyvinylidene difluoride membrane, and probed with the antibodies indicated at the right. Note that all cell lines and treatments were run on gels, probed with antibody, and exposed to film at the same time to allow for accurate and quantitative comparison between cell lines for a given antibody. GAPDH served as a loading control. (B) HCT116 cells untreated or treated with 10 μM 5-azadC (aza) for 48 h or 10 μg/ml bleomycin (bleo) for 4 h. (C) ATM-deficient cells show induction of the active phosphorylated form of CHK1 (phospho-Ser317) in response to 5-azadC treatment (10 μM), demonstrating involvement of the ATR pathway in response to 5-azadC-mediated DNA damage. Equal microgram amounts of whole-cell extract from EBS and YZ5 cells treated with 5-azadC for 0, 24, 48, and 72 h were used as described in panel A. pS, phospho-serine; pT, phospho-threonine. Hrs tx, hours of treatment; untx, untreated.
FIG. 5.
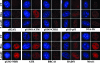
5-azadC treatment induces characteristic relocalization of DNA damage response proteins. Immunofluorescence staining of HeLa cells that were mock treated (UT), treated with 10 μM 5-azadC for 48 h, or treated with 10 μg/ml bleomycin (bleo) for 4 h. Cells were then fixed and stained with the indicated antibodies (red staining) and for DNA (blue staining), and representative images were collected on an upright fluorescence microscope at a magnification of ×100. Note the formation of foci in drug-treated cells and the induction of phosphorylated (active) forms of select proteins.
FIG. 6.
DNMTs relocalize in the presence of 5-azadC, and DNMT1 colocalizes with γ-H2AX DNA damage foci in 5-azadC-treated cells. (A) HeLa cells were transfected with GFP-DNMT1 or GFP-Dnmt3b1. Cells were then mock treated (untreated) or were treated with 10 μM 5-azadC for 48 h, fixed, and then examined for localization of GFP signal. Both GFP-DNMTs localize diffusely throughout the nucleoplasm and are also concentrated in DAPI-dense regions corresponding to heterochromatin in untreated cells (particularly Dnmt3b; green staining), as has been shown previously by us along with other investigators. Upon 5-azadC treatment, DNMT1 and Dnmt3b1 relocalize, becoming aggregated into more discrete foci and absent from DAPI-dense heterochromatin regions. 5-azadC-treated cells were also stained for γ-H2AX (red staining), showing the formation of characteristic foci in cells with damaged DNA. Overlaid images (far right) of the red and green signals show that DNMT1 is highly colocalized with γ-H2AX (yellow signal). (B) DNA staining of untreated and 5-azadC-treated HeLa cells to demonstrate that there is no gross disruption of heterochromatin or nuclear morphology in 5-azadC-treated cells. (C) Colocalization of activated ATM (phosphorylated at serine 1981) and γ-H2AX in 5-azadC-treated HeLa cells. Bar, 5 μm.
FIG. 7.
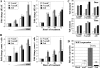
Other clinically relevant nucleoside inhibitors of DNA methylation induce DNA damage in a manner related to their ability to demethylate genes. (A) Direct comparison of the ability of 5-azaC, 5-azadC, and ZEB to induce formation of γ-H2AX foci in HCT116 cells in a dose-dependent (left) and time-dependent (right) manner. Doses (indicated by the wedge) of 5-azaC and 5-azadC used were 0.1, 1.0, and 10 μM, while 50, 250, and 500 μM ZEB was used since it is less potent at inducing demethylation of aberrantly methylated genes. (B) Time- and dose-dependent induction of strand breaks in HCT116 cells treated with the same three drugs as monitored by comet assay. Values are the average of three independent determinations. (C) Calculation of the tail moment, a measure of the degree of DNA damage, from the data in panel B. The upper panel shows the tail moments for the dose-response experiment, and the lower panel shows the tail moments for the time course. −, mock-treated cells or zero hours. (D) Quantitative RT-PCR analysis for expression of the WIF1 gene, which is densely hypermethylated in parental HCT116 cells. RNA prepared from HCT116 cells treated with 10 μM 5-azaC, 10 μM 5-azadC, or 250 μM ZEB was reverse transcribed and PCR amplified with primers for WIF1 and GAPDH. WIF1 expression was normalized to GAPDH expression as described in Materials and Methods, and relative expression of treated versus untreated cells was determined by the ratio of normalized expression values for the treated cells to the normalized expression value of the calibrator (untreated cells) All drug treatments are significantly elevated over values of the mock-treated cells (paired t test, P < 0.04).
FIG. 8.
Altered cellular responses of HCT116 1KO cells when exposed to other genotoxic agents. (A to D) MTT cell viability assays in parental HCT116 and 1KO cells after exposure to increasing doses of doxorubicin, hydroxyurea, bleomycin, and UV light. All values, presented as the percent viability relative to mock-treated cells, are the average of quadruplicate experiments, and the error bar is the standard deviation. Doxorubicin, hydroxyurea, and bleomycin treatments were for 24 h. (E) CHK1 activation following treatment of parental and 1KO HCT116 cells with 1 μM doxorubicin for the indicated times (in minutes), monitored by quantitative Western blotting. Equal amounts of whole-cell extract were used at each time point, and Western blotting was performed with the antibodies indicated at the right. Thin and thick arrows denote unmodified and phosphorylated forms of CHK1, respectively. (F) Representative cell cycle profiles of HCT116 and 1KO cells exposed to 1 μM doxorubicin for 24 h. The percentage of cells in each phase of the cell cycle is given above the G1, S, and G2/M peaks. Parental HCT116 cells arrest predominantly in G2/M and also in G1, while the G2/M and G1 checkpoints appear to be defective in 1KO cells after doxorubicin treatment. Untx, untreated; tx, treatment; Dox, doxorubicin.
FIG. 9.
DNMT1 interacts with CHK1 and influences its subcellular distribution after DNA damage. (A) Demonstration that DNMT1 interacts with CHK1 in reciprocal coimmunoprecipitations. In the top panel, a CHK1 antibody (Ab) was used in the immunoprecipitation (IP), followed by Western blotting (WB) with a DNMT1 antibody. Immunoprecipitation with PCNA and p53 antibodies served as positive controls since they are known to interact with DNMT1, while preimmune IgG served as a negative control. In the bottom panel DNMT1 was used as the immunoprecipitating antibody followed by Western blotting with either CHK1 or CHK2 antibody. DNMT1 does not interact with CHK2 under these conditions. (B) Validation that the DNMT1 antibody used in panel A does indeed immunoprecipitate endogenous DNMT1 from HeLa nuclear extract. Untreated HeLa nuclear extract was used for panels A and B. (C) The DNMT1-CHK1 interaction is enhanced after DNA damage. HeLa cells were mock transfected (−) or transfected with GFP-DNMT1 and FLAG-CHK1. After 24 h, doxorubicin (Dox) was added to the cultures as indicated (+), and whole-cell extract was prepared 24 h later and used in immunoprecipitations with a DNMT1 antibody. CHK1 was detected in Western blotting with an antibody directed against FLAG (upper panel). Expression levels of DNMT1 and CHK1 were equal between untreated and drug-treated cultures (input). (D) Subcellular fractionation of untreated and 5-azadC-treated parental (WT) and 1KO HCT116 cells. Each cell line was mock treated (−) or treated with 10 μM 5-azadC for 48 h (+), followed by isolation of the cytoplasmic (S1/Cyto), soluble nuclear (S2/Nu-S), and chromatin-enriched (P/Chrom) fractions. Each protein fraction was used for Western blotting with the antibodies shown at the left. (E) Validation of the fractionation protocol for HCT116 cells by showing the expected localization of Ku70, GAPDH, and Pol II in the nuclear, cytoplasmic, and chromatin-enriched fractions, respectively. Similar results were obtained with the 1KO cells (data not shown).
Similar articles
- High DNA methyltransferase 3B expression mediates 5-aza-deoxycytidine hypersensitivity in testicular germ cell tumors.
Beyrouthy MJ, Garner KM, Hever MP, Freemantle SJ, Eastman A, Dmitrovsky E, Spinella MJ. Beyrouthy MJ, et al. Cancer Res. 2009 Dec 15;69(24):9360-6. doi: 10.1158/0008-5472.CAN-09-1490. Cancer Res. 2009. PMID: 19951990 Free PMC article. - Consequences of combining siRNA-mediated DNA methyltransferase 1 depletion with 5-aza-2'-deoxycytidine in human leukemic KG1 cells.
Vispé S, Deroide A, Davoine E, Desjobert C, Lestienne F, Fournier L, Novosad N, Bréand S, Besse J, Busato F, Tost J, De Vries L, Cussac D, Riond J, Arimondo PB. Vispé S, et al. Oncotarget. 2015 Jun 20;6(17):15265-82. doi: 10.18632/oncotarget.3317. Oncotarget. 2015. PMID: 25948775 Free PMC article. - Cytotoxicity of 5-Aza-2'-deoxycytidine against gastric cancer involves DNA damage in an ATM-P53 dependent signaling pathway and demethylation of P16(INK4A).
Liu J, Xie YS, Wang FL, Zhang LJ, Zhang Y, Luo HS. Liu J, et al. Biomed Pharmacother. 2013 Feb;67(1):78-87. doi: 10.1016/j.biopha.2012.10.015. Epub 2012 Nov 19. Biomed Pharmacother. 2013. PMID: 23201008 - The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.
Smith J, Tho LM, Xu N, Gillespie DA. Smith J, et al. Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review. - Antineoplastic activity of the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine in anaplastic large cell lymphoma.
Hassler MR, Klisaroska A, Kollmann K, Steiner I, Bilban M, Schiefer AI, Sexl V, Egger G. Hassler MR, et al. Biochimie. 2012 Nov;94(11):2297-307. doi: 10.1016/j.biochi.2012.05.029. Epub 2012 Jun 9. Biochimie. 2012. PMID: 22687603 Free PMC article. Review.
Cited by
- DNA methyltransferases in hematological malignancies.
Hoang NM, Rui L. Hoang NM, et al. J Genet Genomics. 2020 Jul 20;47(7):361-372. doi: 10.1016/j.jgg.2020.04.006. Epub 2020 Jul 24. J Genet Genomics. 2020. PMID: 32994141 Free PMC article. Review. - DNA-dependent protein kinase (DNA-PK)-deficient human glioblastoma cells are preferentially sensitized by Zebularine.
Meador JA, Su Y, Ravanat JL, Balajee AS. Meador JA, et al. Carcinogenesis. 2010 Feb;31(2):184-91. doi: 10.1093/carcin/bgp284. Epub 2009 Nov 23. Carcinogenesis. 2010. PMID: 19933707 Free PMC article. - A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines.
Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N, Krushel L, Aukerman SL, Heise C, MacBeth KJ. Hollenbach PW, et al. PLoS One. 2010 Feb 2;5(2):e9001. doi: 10.1371/journal.pone.0009001. PLoS One. 2010. PMID: 20126405 Free PMC article. - Drugs Targeting Epigenetic Modifications and Plausible Therapeutic Strategies Against Colorectal Cancer.
Patnaik S, Anupriya. Patnaik S, et al. Front Pharmacol. 2019 Jun 6;10:588. doi: 10.3389/fphar.2019.00588. eCollection 2019. Front Pharmacol. 2019. PMID: 31244652 Free PMC article. Review. - Targeted gene therapy of xeroderma pigmentosum cells using meganuclease and TALEN™.
Dupuy A, Valton J, Leduc S, Armier J, Galetto R, Gouble A, Lebuhotel C, Stary A, Pâques F, Duchateau P, Sarasin A, Daboussi F. Dupuy A, et al. PLoS One. 2013 Nov 13;8(11):e78678. doi: 10.1371/journal.pone.0078678. eCollection 2013. PLoS One. 2013. PMID: 24236034 Free PMC article.
References
- Ai, L., Q. Tao, S. Zhong, C. R. Fields, W.-J. Kim, M. W. Lee, Y. Cui, K. D. Brown, and K. D. Robertson. 2006. Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis 271341-1348. - PubMed
- Bachman, K. E., M. R. Rountree, and S. B. Baylin. 2001. Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J. Biol. Chem. 27632282-32287. - PubMed
- Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421499-506. - PubMed
- Barlow, C., M. Liyanage, P. B. Moens, M. Tarsounas, K. Nagashima, K. Brown, S. Rottinghaus, S. P. Jackson, D. Tagle, T. Ried, and A. Wynshaw-Boris. 1998. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 1254007-4017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA116028/CA/NCI NIH HHS/United States
- R01 CA116028/CA/NCI NIH HHS/United States
- R01 CA114229/CA/NCI NIH HHS/United States
- K22CA084535/CA/NCI NIH HHS/United States
- R01CA102289/CA/NCI NIH HHS/United States
- K22 CA084535/CA/NCI NIH HHS/United States
- R01 CA102289/CA/NCI NIH HHS/United States
- R01CA114229/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous