Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility - PubMed (original) (raw)
Review
. 2008 Jan;190(2):463-75.
doi: 10.1128/JB.01418-07. Epub 2007 Nov 9.
Affiliations
- PMID: 17993515
- PMCID: PMC2223684
- DOI: 10.1128/JB.01418-07
Review
Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility
Alan J Wolfe et al. J Bacteriol. 2008 Jan.
No abstract available
Figures
FIG. 1.
Flagellar assembly. Best studied in the enterics (Escherichia coli and Salmonella enterica serovar Typhimurium), the flagellum is comprised of three major substructures: the basal body, the hook, and the filament. These substructures are assembled in order (reviewed in reference 55). The first step in assembly appears to be the insertion into the inner membrane (IM) of the MS ring and proximal rod (composed entirely of FliF subunits) because this insertion can occur independently of all other flagellar components. The second and third steps, assembly of a TSS export apparatus and the switching device, including FliG, occur within the IM and at the cytoplasmic face of the MS ring, respectively. The fourth step, assembly first of the rod and then of the P- and L-ring components, depends upon the presence of both the assembled export apparatus and the switch. Assembly of the distal rod (as well as the hook and filament) depends upon the TSS export apparatus. Secretion of nascent subunits is assisted by a subunit-specific chaperone and depends upon a flagellum-specific ATPase. The first subunits secreted through this TTS apparatus complete the rod, which now bridges the IM, peptidoglycan (PG), and outer membrane (OM). In contrast, assembly of the P ring into the PG and the L ring into the OM depends upon the general secretory apparatus. Following this assembly process, the completed basal body can now serve as a channel through which subsequent proteins travel across the envelope to their assembly site located distal to the OM. First secreted through this envelope-spanning channel are dozens of hook subunits, which are polymerized at the distal end of the basal body. Next secreted and assembled at the distal end of the hook are three HAPs, which serve as adaptors between the larger hook and smaller filament diameters. Two of these HAPs, FlgK and FlgL, bind to their chaperone FlgN and inhibit its ability to activate flgM translation; their secretion relieves this inhibition. This permits increased synthesis of FlgM, the anti-sigma factor for the flagellum-specific sigma factor σ28. Secretion of FlgM relieves inhibition of σ28, which now becomes free to transcribe class III genes, including fliC, which encodes flagellin. Thousands of these flagellin subunits are secreted and assembled between the HAPs to produce the filament. Once the filament is assembled, the HAPs and FlgM are no longer secreted and they resume their inhibitory roles. The result is a semirigid helix strong enough to enable propulsion through a viscous environment. Propulsion occurs because the rod, hook, and filament rotate. Rotation is driven by energy generated by proton (H+) flow across the IM via a PG-anchored proton channel (Mot) that interacts with the switching device. Although the role of FliL is not understood, this protein is membrane bound and associated with the basal body complex (3, 55).
FIG. 2.
Fundamental c-di-GMP pathway. A DGC containing the conserved GGDEF domain catalyzes the synthesis of c-di-GMP from two GTP molecules with the release of pyrophosphate (PPi). A PDE containing the conserved EAL or HD-GYP domain catalyzes the degradation of c-di-GMP to linear diguanylate (l-di-GMP). Together, these two catalytic activities set the steady-state level of c-di-GMP, which can bind to a protein with the conserved PilZ or PelD domain or another as-yet-unidentified domain (X) (21).
FIG. 3.
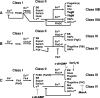
Schematic of transcription hierarchies, as determined for (A) the Enterobacteriaceae (1, 15, 25, 78, 94), (B) Caulobacter (24, 68, 109), and (C) the vibrios (and pseudomonads) (22, 62, 77). Arrows indicate induction, while a straight line in place of the arrowhead indicates inhibition. Note that the hierarchy for the Enterobacteriaceae includes c-di-GMP-associated proteins YcgR and YhjH, which are known to be encoded by genes within the flagellar regulon in Salmonella. The sigma factors at the top of the hierarchies for Caulobacter and vibrio/pseudomonad species are as yet undefined. The levels at which c-di-GMP impacts transcription for V. cholerae (cdgF overexpression; written as c-di-GMP in the figure), V. parahaemolyticus (ScrC/G), and P. putida (MorA) are not known, but the protein that is affected is indicated as an aid for the reader. BB, basal body; Mot, motor proteins; anti-σ28, FlgM; -P, phosphorylated.
FIG. 4.
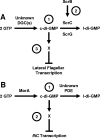
Working model for how c-di-GMP influences flagellar transcription. (A) Scr pathway of V. parahaemolyticus. (Label 1) The PDEs ScrC and ScrG and unidentified DGCs set the steady-state levels of c-di-GMP. (Label 2) ScrB, localized to the periplasm, receives a signal that causes ScrB to modulate ScrA activity. (Label 3) c-di-GMP binds to an unknown c-di-GMP-binding protein, X, to directly or indirectly inhibit lateral flagellar gene transcription. The role of ScrA is as yet unknown and is not included here. The net effect of Scr activity is to decrease c-di-GMP levels and, thus, increase lateral gene transcription. (B) P. putida MorA model. (Label 1) The levels of c-di-GMP are set through the activities of the putative DGC MorA and a hypothetical PDE. (Label 2) c-di-GMP together with a c-di-GMP-binding protein may directly or indirectly inhibit fliC transcription. Thus, the consequence of MorA activity is to decrease fliC transcription. l-di-GMP, linear diguanylate.
FIG. 5.
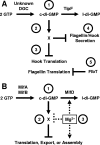
Working models for how c-di-GMP influences flagellar assembly. (A) C. crescentus TipF. (Label 1) An unknown DGC and the PDE TipF control the level of c-di-GMP. (Label 2) c-di-GMP binds to a c-di-GMP-binding protein, X, to directly or indirectly inhibit hook translation (or stability) and flagellin translation (label 3) and the secretion of both (label 4). FlbT independently inhibits flagellin translation (label 5). The net effect of TipF activity is to enhance hook and flagellin translation and secretion. (B) Mif model in V. fischeri. (Label 1) The DGCs MifA and MifB and the PDE MifD set the steady-state levels of c-di-GMP, which binds to an unknown c-di-GMP-binding protein, X (label 2). This complex interferes with the translation, export, and/or assembly of very early flagellar components. (Label 3) Mg2+ inhibits this process at some step downstream of c-di-GMP synthesis, e.g., activation of MifD activity or inhibition of the c-di-GMP-binding protein. l-di-GMP, linear diguanylate.
FIG. 6.
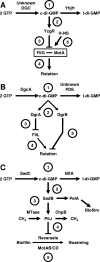
Working models for how c-di-GMP influences flagellar function. (A) YhjH/YcgR model for E. coli and S. enterica. (Label 1) The PDE YhjH and an unidentified DGC set the steady-state levels of c-di-GMP, which (label 2) binds to YcgR. (Label 3) This complex interferes with the ability of cells to properly insert the MotAB energy transduction complex next to the switching device component FliG in the completed basal body, resulting in (label 4) paralyzed flagella. (Label 5) The nucleoid protein H-NS antagonizes this effect by stabilizing the FliG-MotA interaction. The net effect of YcgR activity is to impair rotation, an activity opposed by YhjH. (B) DgcA/DgrAB model for C. crescentus. (Label 1) The DGC DgcA and an unidentified PDE set the steady-state levels of c-di-GMP, which (label 2) binds to DgrA and DgrB. (Label 3) Overexpression of DgrA decreases the steady-state levels of FliL. (Label 4) Since fliL mutants assemble paralyzed flagella, FliL may be integral to DgrA-dependent inhibition of rotation. (Label 5) Like DgrA, overexpression of DgrB inhibits flagellar rotation although it has no effect on FliL. The net effect of DgcA, DgrA, and DgrB is to impair rotation. (C) SadC-BifA model for P. aeruginosa. (Label 1) The DGC SadC and the PDE BifA set the steady-state levels of c-di-GMP, which (label 2) modulates the activity of SadB via a presently unknown mechanism. Also not understood is how SadB influences the activity of (label 3) PelA and the rest of the EPS biosynthetic pathway or (label 4) the methylation state of the chemoreceptor PilJ or (label 5) how PilJ controls the reversal rate, a process that also includes the energy transduction complexes MotAB and MotCD. The net result of SadC and SadB activity is to decrease the reversals necessary for swarming. l-di-GMP, linear diguanylate.
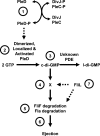
FIG. 7.
Working model for how c-di-GMP influences flagellar ejection. The DGC PleD controls flagellar ejection. (Label 1) Activation of PleD depends upon phosphorylation, which is controlled by two sensor kinases, DivJ and PleC. (Label 2) Phosphorylated PleD (PleD-P) dimerizes, and these dimers can now be polarly localized. (Label 3) The dimer form of PleD is now active as a DGC, and it and an unidentified PDE set the steady-state levels of c-di-GMP. (Label 4) c-di-GMP binds to an unknown c-di-GMP-binding protein, which (label 5) helps to destabilize FliF and possibly another flagellar protein(s) in the proximity of FliF. (Label 6) Destabilization of this protein(s) leads to ejection. (Label 7) FliL appears to be a component of the PleD pathway, but its position in that pathway remains uncertain. l-di-GMP, linear diguanylate.
Similar articles
- Reciprocal c-di-GMP signaling: Incomplete flagellum biogenesis triggers c-di-GMP signaling pathways that promote biofilm formation.
Wu DC, Zamorano-Sánchez D, Pagliai FA, Park JH, Floyd KA, Lee CK, Kitts G, Rose CB, Bilotta EM, Wong GCL, Yildiz FH. Wu DC, et al. PLoS Genet. 2020 Mar 16;16(3):e1008703. doi: 10.1371/journal.pgen.1008703. eCollection 2020 Mar. PLoS Genet. 2020. PMID: 32176702 Free PMC article. - Under Elevated c-di-GMP in Escherichia coli, YcgR Alters Flagellar Motor Bias and Speed Sequentially, with Additional Negative Control of the Flagellar Regulon via the Adaptor Protein RssB.
Nieto V, Partridge JD, Severin GB, Lai RZ, Waters CM, Parkinson JS, Harshey RM. Nieto V, et al. J Bacteriol. 2019 Dec 6;202(1):e00578-19. doi: 10.1128/JB.00578-19. Print 2019 Dec 6. J Bacteriol. 2019. PMID: 31611290 Free PMC article. - PP4397/FlgZ provides the link between PP2258 c-di-GMP signalling and altered motility in Pseudomonas putida.
Wirebrand L, Österberg S, López-Sánchez A, Govantes F, Shingler V. Wirebrand L, et al. Sci Rep. 2018 Aug 15;8(1):12205. doi: 10.1038/s41598-018-29785-w. Sci Rep. 2018. PMID: 30111852 Free PMC article. - Taming the flagellar motor of pseudomonads with a nucleotide messenger.
Ma GL, Chandra H, Liang ZX. Ma GL, et al. Environ Microbiol. 2020 Jul;22(7):2496-2513. doi: 10.1111/1462-2920.15036. Epub 2020 May 5. Environ Microbiol. 2020. PMID: 32329141 Review. - Cyclic di-GMP, an established secondary messenger still speeding up.
Römling U. Römling U. Environ Microbiol. 2012 Aug;14(8):1817-29. doi: 10.1111/j.1462-2920.2011.02617.x. Epub 2011 Oct 31. Environ Microbiol. 2012. PMID: 22040037 Review.
Cited by
- Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1.
Newell PD, Yoshioka S, Hvorecny KL, Monds RD, O'Toole GA. Newell PD, et al. J Bacteriol. 2011 Sep;193(18):4685-98. doi: 10.1128/JB.05483-11. Epub 2011 Jul 15. J Bacteriol. 2011. PMID: 21764921 Free PMC article. - Bordetella bronchiseptica Diguanylate Cyclase BdcA Regulates Motility and Is Important for the Establishment of Respiratory Infection in Mice.
Belhart K, Gutierrez MP, Zacca F, Ambrosis N, Cartelle Gestal M, Taylor D, Dahlstrom KM, Harvill ET, O'Toole GA, Sisti F, Fernández J. Belhart K, et al. J Bacteriol. 2019 Aug 8;201(17):e00011-19. doi: 10.1128/JB.00011-19. Print 2019 Sep 1. J Bacteriol. 2019. PMID: 31209073 Free PMC article. - Identification of novel factors involved in modulating motility of Salmonella enterica serotype typhimurium.
Bogomolnaya LM, Aldrich L, Ragoza Y, Talamantes M, Andrews KD, McClelland M, Andrews-Polymenis HL. Bogomolnaya LM, et al. PLoS One. 2014 Nov 4;9(11):e111513. doi: 10.1371/journal.pone.0111513. eCollection 2014. PLoS One. 2014. PMID: 25369209 Free PMC article. - Quantitative confocal microscopy and calibration for measuring differences in cyclic-di-GMP signalling by bacteria on biomedical hydrogels.
Blacutt J, Lan Z, Cosgriff-Hernandez EM, Gordon VD. Blacutt J, et al. R Soc Open Sci. 2021 Jan 6;8(1):201453. doi: 10.1098/rsos.201453. eCollection 2021 Jan. R Soc Open Sci. 2021. PMID: 33614081 Free PMC article. - Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis.
Bobrov AG, Kirillina O, Ryjenkov DA, Waters CM, Price PA, Fetherston JD, Mack D, Goldman WE, Gomelsky M, Perry RD. Bobrov AG, et al. Mol Microbiol. 2011 Jan;79(2):533-51. doi: 10.1111/j.1365-2958.2010.07470.x. Epub 2010 Dec 3. Mol Microbiol. 2011. PMID: 21219468 Free PMC article.
References
- Aldridge, P., and K. T. Hughes. 2002. Regulation of flagellar assembly. Curr. Opin. Microbiol. 5160-165. - PubMed
- Aldridge, P., and U. Jenal. 1999. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol. Microbiol. 32379-391. - PubMed
- Aldridge, P., J. E. Karlinsey, and K. T. Hughes. 2003. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol. Microbiol. 491333-1345. - PubMed
- Aldridge, P., R. Paul, P. Goymer, P. Rainey, and U. Jenal. 2003. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 471695-1708. - PubMed
- Amikam, D., and M. Y. Galperin. 2006. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 223-6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases