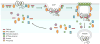G protein-coupled receptor sorting to endosomes and lysosomes - PubMed (original) (raw)
Review
G protein-coupled receptor sorting to endosomes and lysosomes
Adriano Marchese et al. Annu Rev Pharmacol Toxicol. 2008.
Abstract
The heptahelical G protein-coupled receptors (GPCRs) belong to the largest family of cell surface signaling receptors encoded in the human genome. GPCRs signal to diverse extracellular stimuli and control a vast number of physiological responses, making this receptor class the target of nearly half the drugs currently in use. In addition to rapid desensitization, receptor trafficking is crucial for the temporal and spatial control of GPCR signaling. Sorting signals present in the intracytosolic domains of GPCRs regulate trafficking through the endosomal-lysosomal system. GPCR internalization is mediated by serine and threonine phosphorylation and arrestin binding. Short, linear peptide sequences including tyrosine- and dileucine-based motifs, and PDZ ligands that are recognized by distinct endocytic adaptor proteins also mediate internalization and endosomal sorting of GPCRs. We present new data from bioinformatic searches that reveal the presence of these types of sorting signals in the cytoplasmic tails of many known GPCRs. Several recent studies also indicate that the covalent modification of GPCRs with ubiquitin serves as a signal for internalization and lysosomal sorting, expanding the diversity of mechanisms that control trafficking of mammalian GPCRs.
Figures
Figure 1
Model of GPCR recruitment to clathrin-coated pits (CCPs). The clathrin adaptor AP-2 is first recruited to the plasma membrane at sites enriched in PIP2 and facilitates the recruitment of more AP-2, clathrin, and alternate clathrin adaptors. Many activated GPCRs are phosphorylated and bind arrestins, which facilitates recruitment into the nascent CCPs. Other GPCRs harbor short linear peptide sequences or are modified with ubiquitin and recognized by alternate clathrin adaptors. The newly formed CCPs gradually invaginate and pinch off from the plasma membrane through the actions of the GTPase dynamin to form a vesicle. Many other accessory proteins and alternate clathrin adaptors are also present in the nascent and forming CCPs.
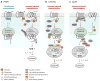
Figure 2
G protein–coupled receptor trafficking within the endosomal-lysosomal system. Several studies indicate that distinct mechanisms control trafficking of different mammalian GPCRs. (a) PAR1 displays constitutive and agonist-induced internalization that occur independent of arrestins and is differentially regulated by ubiquitination. Constitutive internalization of PAR1 requires AP-2 and is negatively regulated by ubiquitination. Activated PAR1 is phosphorylated, rapidly internalized, and sorted from endosomes to lysosomes through a SNX1-dependent pathway, which is independent of HRS and TSG101, a component of the ESCRT-I machinery. Whether PAR1 enters MVBs (multivesicular bodies) or sorts directly to lysosomes is not known. (b) Activated CXCR4 is ubiquitinated at the plasma membrane by the E3 ubiquitin ligase AIP4. Ubiquitination functions as an endosomal sorting signal and is not required for CXCR4 internalization. Ubiquitinated CXCR4 is concentrated on HRS-positive microdomains together with AIP4. AIP4 mediates ubiquitination of HRS following CXCR4 activation, which is critical for MVB sorting. CISK phosphorylates and inhibits AIP4 activity and thereby inhibits endosomal sorting of CXCR4. VPS4 also regulates the ubiquitination status of CXCR4 and MVB sorting. (c) Agonist induces rapid phosphorylation and ubiquitination of the β2AR. Arrestin-3 is ubiquitinated by MDM2 and recruited to activated β2AR, a process required for β2AR internalization. Once internalized, β2AR are dephosphorylated and rapidly recycled back to the plasma membrane through a pathway that involves EBP50/NHERF, NSF, and HRS. Whether deubiquitination of β2AR is involved in the regulated mode of receptor recycling is not known. After prolonged agonist exposure, activated β2AR sorts from endosome to lysosomes and are degraded through an ubiquitin-dependent mechanism.
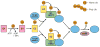
Figure 3
The ubiquitin modification of proteins. Ubiquitin (Ub) is attached to its target protein by the sequential action of E1, E2, and E3 enzymes. The E1 (activating enzyme) first activates ubiquitin in an ATP-dependent reaction by forming a thioester bond at its active-site cysteine with the COOH-terminus of ubiquitin. Ubiquitin is then transferred to the active site cysteine of the E2 (conjugating enzyme). The last step is catalyzed by either an E2 with the help of an E3 (RING-finger) or directly by an E3 (HECT-domain), leading to the transfer of ubiquitin to an epsilon amino group of a lysine residue on the target protein forming an isopeptide bond with the C-terminal glycine of ubiquitin. A single ubiquitin can be attached to proteins (Mono Ub) at a single and/or multiple lysine residues on the target protein. Alternatively multiple ubiquitins can be attached to one another forming poly Ub chains (Poly Ub), typically via lysine 48 or 63 linkages. Ubiquitin is removed from target proteins by the action of deubiquitinating enzymes (DUBs).
Similar articles
- Regulation of GPCR Trafficking by Ubiquitin.
Kennedy JE, Marchese A. Kennedy JE, et al. Prog Mol Biol Transl Sci. 2015;132:15-38. doi: 10.1016/bs.pmbts.2015.02.005. Epub 2015 Mar 25. Prog Mol Biol Transl Sci. 2015. PMID: 26055053 Free PMC article. Review. - Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: Diverse pathways for G-protein-coupled receptor destruction and beyond.
Dores MR, Trejo J. Dores MR, et al. Traffic. 2019 Feb;20(2):101-109. doi: 10.1111/tra.12619. Epub 2018 Nov 18. Traffic. 2019. PMID: 30353650 Free PMC article. Review. - Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling.
Marchese A, Trejo J. Marchese A, et al. Cell Signal. 2013 Mar;25(3):707-16. doi: 10.1016/j.cellsig.2012.11.024. Epub 2012 Nov 29. Cell Signal. 2013. PMID: 23201781 Free PMC article. Review. - Signals for sorting of transmembrane proteins to endosomes and lysosomes.
Bonifacino JS, Traub LM. Bonifacino JS, et al. Annu Rev Biochem. 2003;72:395-447. doi: 10.1146/annurev.biochem.72.121801.161800. Epub 2003 Mar 6. Annu Rev Biochem. 2003. PMID: 12651740 Review. - Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis.
Wolfe BL, Trejo J. Wolfe BL, et al. Traffic. 2007 May;8(5):462-70. doi: 10.1111/j.1600-0854.2007.00551.x. Epub 2007 Mar 21. Traffic. 2007. PMID: 17376169 Review.
Cited by
- Diversity and impact of rare variants in genes encoding the platelet G protein-coupled receptors.
Jones ML, Norman JE, Morgan NV, Mundell SJ, Lordkipanidzé M, Lowe GC, Daly ME, Simpson MA, Drake S, Watson SP, Mumford AD; UK GAPP study group. Jones ML, et al. Thromb Haemost. 2015 Apr;113(4):826-37. doi: 10.1160/TH14-08-0679. Epub 2015 Jan 8. Thromb Haemost. 2015. PMID: 25567036 Free PMC article. - GPR35 as a Novel Therapeutic Target.
Mackenzie AE, Lappin JE, Taylor DL, Nicklin SA, Milligan G. Mackenzie AE, et al. Front Endocrinol (Lausanne). 2011 Nov 9;2:68. doi: 10.3389/fendo.2011.00068. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654822 Free PMC article. - Adenosine receptors and membrane microdomains.
Lasley RD. Lasley RD. Biochim Biophys Acta. 2011 May;1808(5):1284-9. doi: 10.1016/j.bbamem.2010.09.019. Epub 2010 Oct 1. Biochim Biophys Acta. 2011. PMID: 20888790 Free PMC article. Review. - RNA interference screen for RGS protein specificity at muscarinic and protease-activated receptors reveals bidirectional modulation of signaling.
Laroche G, Giguère PM, Roth BL, Trejo J, Siderovski DP. Laroche G, et al. Am J Physiol Cell Physiol. 2010 Sep;299(3):C654-64. doi: 10.1152/ajpcell.00441.2009. Epub 2010 Jun 23. Am J Physiol Cell Physiol. 2010. PMID: 20573995 Free PMC article. - G-protein coupled receptor, PI3K and Rho signaling pathways regulate the cascades of Tau and amyloid-β in Alzheimer's disease.
Desale SE, Chidambaram H, Chinnathambi S. Desale SE, et al. Mol Biomed. 2021 Jun 10;2(1):17. doi: 10.1186/s43556-021-00036-1. Mol Biomed. 2021. PMID: 35006431 Free PMC article. Review.
References
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:1–9. - PubMed
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–55. Excellent comprehensive review on the ubiquitin-dependent endosomal sorting required for transport (ESCRT) machinery. - PubMed
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–17. - PubMed
- Hirsch JA, Schubert C, Gurevich V, Sigler PA. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97:257–69. - PubMed
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, et al. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL073328/HL/NHLBI NIH HHS/United States
- R01 GM075159-03/GM/NIGMS NIH HHS/United States
- R01 GM090689/GM/NIGMS NIH HHS/United States
- HL 073328/HL/NHLBI NIH HHS/United States
- R01 HL073328-06/HL/NHLBI NIH HHS/United States
- GM 075159/GM/NIGMS NIH HHS/United States
- R01 GM075159/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources