Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis - PubMed (original) (raw)
Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis
Sean L Petersen et al. Cancer Cell. 2007 Nov.
Abstract
A small-molecule mimetic of Smac/Diablo that specifically counters the apoptosis-inhibiting activity of IAP proteins has been shown to enhance apoptosis induced by cell surface death receptors as well as chemotherapeutic drugs. Survey of a panel of 50 human non-small-cell lung cancer cell lines has revealed, surprisingly, that roughly one-quarter of these lines are sensitive to the treatment of Smac mimetic alone, suggesting that an apoptotic signal has been turned on in these cells and is held in check by IAP proteins. This signal has now been identified as the autocrine-secreted cytokine tumor necrosis factor alpha (TNFalpha). In response to autocrine TNFalpha signaling, the Smac mimetic promotes formation of a RIPK1-dependent caspase-8-activating complex, leading to apoptosis.
Figures
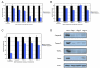
Figure 1. Response of a Panel of Lung Cancer Cell Lines to Smac Mimetic In Vitro and In Vivo
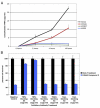
(A) A panel of 50 non-small-carcinoma-cell lung cancer cell lines was tested for responsiveness to a Smac-mimetic treatment alone. IC50s were determined for each cell line based on cell survival as measured by ATP levels in live cells using Cell Titer-glo (Promega). IC50 determination was based on concentrations of compound 3 that yielded half-maximal luminescence relative to untreated cells. (B) Treatment of selected group of cell lines to 100 nM of compound 3 and to compound 4 (negative control compound with similar structure by differing in function group configuration as described in Li et al., 2004). Smac-mimetic-sensitive cell lines (HCC44 and HCC461) and Smac-mimeticresistant cell lines (HCC827 and H2009) were chosen. Each graphical representation for IC50s and cell survival indicates the mean ± SD of at least three independent testing conditions. (C) In vitro pull-down utilizing a biotinylated form of compound 3. The biotinylated compound was able to pull down protein bands at around 50 kDa and at around 70 kDa not seen in control lanes (avidin beads only), which were cut out of the gel and analyzed by mass spectroscopy. Proteins identified are indicated. (D) In vivo response of mouse xenografts of HCC461 cells to compound 3. Harlan athymic nude mice were injected subcutaneously with HCC461 cells in a matrigel randomly separated into treatment groups (n = 5) and given six intravenous injections of compound 3 or saline every other day. Tumors were measured twice perweek until the end of the experiment. In the compound 3 treatment group, 2/5 (40%) remained tumor-free at the end of the experiment. (E) In vivo response of mouse xenografts of a Smac-mimetic-resistant HCC15 cells to compound 3. Conditions were identical to those for HCC461 xenografts. For tumor size measurements, graphical representations indicate the mean ± SEM of five individual samples per condition.
Figure 2. Smac-Mimetic-Mediated Cell Death Displays Caspase Activation, which Can Be Blocked by Caspase Inhibition
Analysis of caspase activation in HCC461 cell lysates following a time course of 100 nM compound 3 treatments. For caspase inhibition, 10 μM z vad fmk (Sigma) was added 1 hr prior to compound 3. (A) Western blot analysis of caspase-3 activation. (B) Western blot analysis of caspase-8 activation. (C) Rescue of Smac mimetic cell death by 10 μM z vad. (D) Long-term survival of cells treated with z vad plus compound 3. Cells were treated as indicated for 5 days to ensure that rescue from cell death was not a transient artifact of z vad treatment. Cell viability was determined using methylene blue staining. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
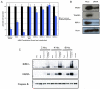
Figure 3. Sirna Candidate Screen Implicates TNFα Signaling as a Requirement for Smac-Mimetic-Mediated Apoptosis
Cells were assayed for the ability of particular transiently transfected individual siRNAs (Dharmacon) to produce a rescue phenotype in HCC461 cells following 100 nM compound 3 treatments. Cell viability was determined by measuring total ATP levels. Each siRNA treatment and corresponding compound treatment (n = 4, per transfection and compound 3 treatment) was normalized to the mock-transfected control to account for cytotoxicity. (A) Caspase-8 and -9 siRNA transfection. (B) TNFR1 siRNA transfection. (C) TNFα siRNA transfection. D) Efficiency of total protein level knockdown per siRNA was determined by western blot. Caspase-8-positive controls in (B) and (3) utilized Dharmacon’s siGENOME SMARTpool predesigned pools of four oligos. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
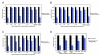
Figure 4. Autocrine TNFα Signaling Is Required for Smac-Mimetic-Mediated Apoptosis
(A) Smac-mimetic-sensitive cell lines (HCC44 and HCC461) and Smac-mimetic-resistant cell lines (HCC827 and H2009) were tested for the presence of TNFα in conditioned cell culture media for each cell line. Samples were removed at the indicated time points and used for quantitative sandwich enzyme immunoassay analysis (R&D Systems) to determine the concentration of TNFα present, as described in the Experimental Procedures section. Sensitive cells were secreting low levels of TNFα in the cell culture medium, while there was no detectable TNFα levels present in resistant cell lines. (B) Pretreatment (1 hr) of neutralizing antibodies (1–2 ug/mL) against TNFα (R&D Systems), TRAIL (Biolegend), and FasL/CD95 (Biolegend) prior to 100 nM compound 3 treatments. Cell viability was determines as previously described. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
Figure 5. Smac-Mimetic-Mediated Apoptosis Is RIPK1 Dependent and Promotes the Formation of a RIPK1-FADD-Caspase 8 Complex in HCC461 Cells
(A) siRNAs targeting known components of the TNF signaling pathway (FADD, TRADD, and RIPK1). RIPK1 dependence was verified by the use of single siRNA oligos targeting different regions of the mRNA. siRNA transfection and viability assays were done as previously described. (B) Western blot analysis of protein levels following siRNA transfection, as described previously. (C) RIPK1, FADD, caspase-8 complex coimmunoprecipitation using caspase-8 antibody (Santa Cruz, SC-6136). Cells were treated in the indicated manner and coimmunoprecipitations were done as described in the Experimental Procedures. Z vad was added as a means of capturing caspase-8 in complex with RIPK1 and FADD by preventing full activation and subsequent dissociation of the complex from caspase-8. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
Figure 6. Other Smac-Mimetic-Sensitive Cells Respond in a Similar Fashion as HCC461 Cells
Cell lines HCC44, MDA-MB-231, and SK MEL-5 were tested for rescue by siRNA transfection and neutralizing antibody pretreatments. siRNAs targeting caspase-8, TNFR1, TNFα, and RIPK1 (Dharmacon siGENOME SMARTpool predesigned pools of four oligos), as well as neutralizing antibody against TNFα (R&D Systems), were able to rescue these cell lines from Smac-mimetic-mediated apoptosis, while caspase-9 siRNA transfection (Dharmacon siGENOME SMARTpool) and pretreatment with TRAIL (Biolegend) neutralizing antibody had no rescue effects. (A) HCC44. (B) MDA-MB-231. (C) SK MEL-5. (D) H2009 cells were shown to respond to 100 nM compound 3 and 100 ng/ml TNFα. siRNA knockdown of caspase-8, TNFR1, and RIPK1 were able to rescue these cells from compound 3/TNFα cotreatment. siRNA transfection and cell viability assays were done as previously described. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
Similar articles
- Doxorubicin sensitizes cancer cells to Smac mimetic via synergistic activation of the CYLD/RIPK1/FADD/caspase-8-dependent apoptosis.
Yang C, Ran Q, Zhou Y, Liu S, Zhao C, Yu X, Zhu F, Ji Y, Du Q, Yang T, Zhang W, He S. Yang C, et al. Apoptosis. 2020 Jun;25(5-6):441-455. doi: 10.1007/s10495-020-01604-6. Apoptosis. 2020. PMID: 32418059 - Receptor-interacting protein kinase 1 is a key mediator in TLR3 ligand and Smac mimetic-induced cell death and suppresses TLR3 ligand-promoted invasion in cholangiocarcinoma.
Lomphithak T, Choksi S, Mutirangura A, Tohtong R, Tencomnao T, Usubuchi H, Unno M, Sasano H, Jitkaew S. Lomphithak T, et al. Cell Commun Signal. 2020 Oct 9;18(1):161. doi: 10.1186/s12964-020-00661-3. Cell Commun Signal. 2020. PMID: 33036630 Free PMC article. - TNF-alpha induces two distinct caspase-8 activation pathways.
Wang L, Du F, Wang X. Wang L, et al. Cell. 2008 May 16;133(4):693-703. doi: 10.1016/j.cell.2008.03.036. Cell. 2008. PMID: 18485876 - Smac mimetics and TNFalpha: a dangerous liaison?
Wu H, Tschopp J, Lin SC. Wu H, et al. Cell. 2007 Nov 16;131(4):655-8. doi: 10.1016/j.cell.2007.10.042. Cell. 2007. PMID: 18022360 Free PMC article. Review. - SMAC Mimetics for the Treatment of Lung Carcinoma: Present Development and Future Prospects.
Pandey R, Bisht P, Wal P, Murti K, Ravichandiran V, Kumar N. Pandey R, et al. Mini Rev Med Chem. 2024;24(14):1334-1352. doi: 10.2174/0113895575269644231120104501. Mini Rev Med Chem. 2024. PMID: 38275029 Review.
Cited by
- Small-molecule activation of the TRAIL receptor DR5 in human cancer cells.
Wang G, Wang X, Yu H, Wei S, Williams N, Holmes DL, Halfmann R, Naidoo J, Wang L, Li L, Chen S, Harran P, Lei X, Wang X. Wang G, et al. Nat Chem Biol. 2013 Feb;9(2):84-9. doi: 10.1038/nchembio.1153. Epub 2012 Dec 23. Nat Chem Biol. 2013. PMID: 23292651 - Induction of Breast Cancer Cell Apoptosis by TRAIL and Smac Mimetics: Involvement of RIP1 and cFLIP.
Holmgren C, Sunström Thörnberg E, Granqvist V, Larsson C. Holmgren C, et al. Curr Issues Mol Biol. 2022 Oct 11;44(10):4803-4821. doi: 10.3390/cimb44100327. Curr Issues Mol Biol. 2022. PMID: 36286042 Free PMC article. - SMAC mimetics induce autophagy-dependent apoptosis of HIV-1-infected macrophages.
Campbell GR, To RK, Zhang G, Spector SA. Campbell GR, et al. Cell Death Dis. 2020 Jul 27;11(7):590. doi: 10.1038/s41419-020-02761-x. Cell Death Dis. 2020. PMID: 32719312 Free PMC article. - NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression.
Liu F, Bardhan K, Yang D, Thangaraju M, Ganapathy V, Waller JL, Liles GB, Lee JR, Liu K. Liu F, et al. J Biol Chem. 2012 Jul 20;287(30):25530-40. doi: 10.1074/jbc.M112.356279. Epub 2012 Jun 5. J Biol Chem. 2012. PMID: 22669972 Free PMC article. - USP11-dependent selective cIAP2 deubiquitylation and stabilization determine sensitivity to Smac mimetics.
Lee EW, Seong D, Seo J, Jeong M, Lee HK, Song J. Lee EW, et al. Cell Death Differ. 2015 Sep;22(9):1463-76. doi: 10.1038/cdd.2014.234. Epub 2015 Jan 23. Cell Death Differ. 2015. PMID: 25613375 Free PMC article.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. - PubMed
- Bhardwaj A, Aggarwal BB. Receptor-mediated choreography of life and death. J. Clin. Immunol. 2003;23:317–332. - PubMed
- Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. - PubMed
- Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA 95471/CA/NCI NIH HHS/United States
- CA70907/CA/NCI NIH HHS/United States
- P50 CA070907/CA/NCI NIH HHS/United States
- CA84971/CA/NCI NIH HHS/United States
- P01 CA095471/CA/NCI NIH HHS/United States
- U01 CA084971/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous