Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex - PubMed (original) (raw)
Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex
Lise Lyck et al. J Histochem Cytochem. 2008 Mar.
Abstract
Reproducible visualization of neurons and glia in human brain is essential for quantitative studies of the cellular changes in neurological disease. However, immunohistochemistry in human brain specimens is often compromised because of prolonged fixation. To select cell lineage-specific antibodies for quantitative studies of neurons and the major types of glia, we used 29 different antibodies, different epitope retrieval methods, and different detection systems to stain tissue arrays of formalin-fixed human brain. The screening pointed at CD45/leukocyte common antigen (LCA), CD68(KP1), 2',3' cyclic nucleotide phosphatase (CNPase), glial fibrillary acidic protein (GFAP), HLA-DR, Ki67, neuronal nuclei (NeuN), p25alpha-antigen, and S100beta as candidates for future cell counting purposes, because these markers visualized specific neuronal and glial cell bodies. However, significant negative correlation between staining result and formalin fixation was observed by blinded scoring of staining for CD45/LCA, CNPase, GFAP, and NeuN in brain specimens fixed by immersion and stored up to 10 years in 4% formalin solution at room temperature, independent of donor sex and postmortem interval. In contrast, improved preservation of NeuN and CNPase staining, and full preservation of GFAP and CD45/LCA staining in tissue fixed by perfusion and stored for up to 3 years in 0.1% paraformaldehyde solution at 4C, indicated that immunohistochemistry can be performed in well-preserved biobank material.
Figures
Figure 1
Visualization of neuronal cell bodies and cell processes vary with the choice of antigen/antibody combination. High-magnification micrographs showing the staining for β-tubulin III (A), microtubule-associated protein-2 (MAP-2) (B), neuronal nuclei (NeuN) (C), neurofilaments (NF) (D), NF [PAN, clone: DA2/FNP7/RmdO20.11 (PAN)] (E), and neuron-specific enolase (NSE) (F) were acquired in layer V of the parietal neocortex from an adult donor (A–D,F: Donor 6 in Table 1; E: Donor 4 in Table 2). Paraffin sections were stained using Dako ChemMate LSAB system, the heat-induced epitope retrieval (HIER) method, and antibody dilutions listed in Table 5. Arrows indicate labeled neuronal cell bodies; arrowheads indicate labeled cell processes. Bar = 20 μm.
Figure 2
Differences in cellular expression of developmentally regulated antigens in fetal and infant tissue. (A,B) Turned on after division-64/Tuc-4 (TOAD-64), (C,D) nestin, (E,F) vimentin, (G,H) platelet-derived growth factor-α receptor (PDGFα-R), (I,J) Nkx2.2, and (K,L) Ki67. Photomicrographs were acquired in the intermediate zone in fetal brain (Donor 1 in Table 1) and in white matter in the brain from an infant (Donor 4 in Table 1). Staining was performed in paraffin sections using the Dako ChemMate LSAB system, the HIER method, and antibody dilutions listed in Table 5. Arrows indicate specifically labeled cells; arrowheads (D-F) indicate specific labeling of vascular structures. Bar = 40 μm.
Figure 3
Visualization of astroglial, oligodendroglial, and microglial cell bodies in the parietal neocortex from an adult donor. (A–C) Staining for astroglial markers showing glial fibrillary acidic protein (GFAP)+ astroglia (A), S100β+ glia (B), and vimentin+ astrocytes and cells of the capillary walls (arrowheads) (C). (D–F) Markers of oligodendroglia and myelin showing myelin basic protein (MBP)+ myelin fibers but no labeled cell bodies (D), 2′3′-cyclic nucleotide 3′-phosphodiesterase (CNPase)+ myelin fibers and round cell bodies (arrows) (E), and round cell bodies labeled by P25 α-antigen/tubulin polymerization promoting protein (p25α) (F). (G–I) Staining for microglia showing CD45+ ramified microglia (G), CD68+ (KP1) microglia showing a more punctuate labeling of their cell processes (H), and HLA-DR labeling the cellular processes (I). Paraffin sections from tissue array A were stained using the Dako ChemMate LSAB system, HIER method, and antibody dilutions listed in Table 5. Micrographs were acquired in neocortical layer VIa in samples from Donor 6 (Table 1). Labeled cell bodies are indicated by arrows. Bar = 20 μm.
Figure 4
Influence of epitope retrieval technique on staining quality. The effect of HIER and proteolytic epitope retrieval (PrER) were tested on stainings for GFAP and MBP in paraffin sections of adult neocortical tissue fixed for 24 hr and 3 months. (A–D) No epitope retrieval (Non), (E–H) pretreatment by PrER with proteinase K (Prot K), and (I–L) HIER by heating in microwave oven in Tris-EGTA buffer, pH 9.0 (TEG buffer). Binding of the primary rabbit-anti-GFAP-antibody (1:1000) or rabbit-anti-MBP-antibody (1:200) was visualized using the Dako LSAB-ChemMate detection system. Photomicrographs were acquired in neocortical layer VI in specimens from Donor 6 (A,B,E,F,I,J) and Donor 8 (C,D,G,H,K,L) from tissue array A (Table 1). Results were superior using epitope retrieval with HIER.
Figure 5
Influence of detection system on staining quality. The visualization of cell bodies and cell processes, illustrated by the visualization of neocortical GFAP+ astrocytes in paraffin sections of adult human brain material fixed for 24 hr (A–C) and 10 years (D–F), depended on the choice of detection system. (A,D) Detection by Dako LSAB-ChemMate detection system. (B,E) Detection by Dako Envision+ peroxidase-labeled polymer. (C,F) Detection by the Vectastain Universal Elite ABC kit. Horseradish peroxidase activity was detected using Dako DAB+-chromogenic kit for 3 × 5 min. Staining was carried out on paraffin sections that were subjected to HIER by heating in a microwave oven in TEG buffer before incubation in rabbit-anti-GFAP-antibody (1:2000). Photomicrographs were acquired in neocortical layers V–VI in specimens from Donor 6 and Donor 10 in tissue array A (Table 1). Results were superior using the Dako Envision+ peroxidase-labeled polymer.
Figure 6
Scoring of immunohistochemical stainings for NeuN, GFAP, CNPase, and CD45. Photomicrographs were acquired in neocortical layer VI in specimens from tissue array B (Table 2) scored as 1 (A,E,I,M), 2 (B,F,J,N), 3 (C,G,K,O), and 4 (D,H,L,P) according to the scale in Table 4. Staining was performed using the HIER method and antibody dilutions in Table 5. Bar = 50 μm.
Figure 7
Correlation between immunohistochemical staining result and storage time in 4% Lillies phosphate-buffered formalin solution (PBFS) at room temperature. (A) NeuN, (B) CNPase, (C) GFAP, and (D) CD45. Specimens from tissue array B were stained using HIER and detected by the Dako Envision+ peroxidase-labeled polymer. Stainings were scored using the scale given in Table 4 and shown in Figure 6, and the mean score (points) and SEM (error bars) were calculated. The data were stratified for sex (A,C,E,G) and PMI (B,D,F,H) and analyzed for correlation using the Spearman r test. The r value is given for each group along with the p value. NS, not significant. *p<0.05; **p<0.01; ***p<0.001.
Figure 8
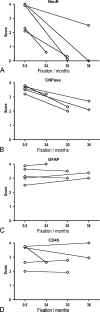
Immunohistochemical staining result in perfusion-fixed brain material. Graphic illustration of changes in immunohistochemical staining results for NeuN (A), CNPase (B), GFAP (C), and CD45 (D) resulting from long-term storage of perfusion fixed brain material in 0.1% paraformaldehyde in 0.15 M Sørensens phosphate buffer, pH 7.4 (PFA) solution at 4C. The stainings were performed on specimens in tissue array B (Donors 2–6 in Table 2), prepared as paraffin sections, stained for NeuN, CNPase, GFAP, and CD45 using HIER, and detected by Dako Envision+. Stainings were scored using the scale given in Table 4 and shown in Figure 6. Data are presented as mean scores of individual specimens (I) at 2 weeks and 3–4 years of fixation. Mean scores of specimens deriving from the same donor are connected by a straight line.
Similar articles
- Immunohistochemical visualization of neurons and specific glial cells for stereological application in the porcine neocortex.
Lyck L, Jelsing J, Jensen PS, Lambertsen KL, Pakkenberg B, Finsen B. Lyck L, et al. J Neurosci Methods. 2006 Apr 15;152(1-2):229-42. doi: 10.1016/j.jneumeth.2005.09.009. Epub 2005 Nov 2. J Neurosci Methods. 2006. PMID: 16269187 - Structural and chemical changes in glial cells in the rat neocortex induced by constant occlusion of the middle cerebral artery.
Kalinichenko SG, Korobtsov AV, Matveeva NY, Pushchin II. Kalinichenko SG, et al. Acta Histochem. 2020 Jul;122(5):151573. doi: 10.1016/j.acthis.2020.151573. Epub 2020 Jun 13. Acta Histochem. 2020. PMID: 32622419 - An empirical analysis of the precision of estimating the numbers of neurons and glia in human neocortex using a fractionator-design with sub-sampling.
Lyck L, Santamaria ID, Pakkenberg B, Chemnitz J, Schrøder HD, Finsen B, Gundersen HJ. Lyck L, et al. J Neurosci Methods. 2009 Sep 15;182(2):143-56. doi: 10.1016/j.jneumeth.2009.06.003. Epub 2009 Jun 9. J Neurosci Methods. 2009. PMID: 19520115 - Cellular reactions of the central nervous system.
Kovacs GG. Kovacs GG. Handb Clin Neurol. 2017;145:13-23. doi: 10.1016/B978-0-12-802395-2.00003-1. Handb Clin Neurol. 2017. PMID: 28987163 Review. - Lysosomal Functions in Glia Associated with Neurodegeneration.
Kreher C, Favret J, Maulik M, Shin D. Kreher C, et al. Biomolecules. 2021 Mar 9;11(3):400. doi: 10.3390/biom11030400. Biomolecules. 2021. PMID: 33803137 Free PMC article. Review.
Cited by
- Fluid preservation in brain banking: a review.
McKenzie AT, Nnadi O, Slagell KD, Thorn EL, Farrell K, Crary JF. McKenzie AT, et al. Free Neuropathol. 2024 Apr 23;5:5-10. doi: 10.17879/freeneuropathology-2024-5373. eCollection 2024 Jan. Free Neuropathol. 2024. PMID: 38690035 Free PMC article. - Immunohistochemical field parcellation of the human hippocampus along its antero-posterior axis.
González-Arnay E, Pérez-Santos I, Jiménez-Sánchez L, Cid E, Gal B, de la Prida LM, Cavada C. González-Arnay E, et al. Brain Struct Funct. 2024 Mar;229(2):359-385. doi: 10.1007/s00429-023-02725-9. Epub 2024 Jan 5. Brain Struct Funct. 2024. PMID: 38180568 Free PMC article. - Neocortex neurogenesis and maturation in the African greater cane rat.
Mustapha O, Grochow T, Olopade J, Fietz SA. Mustapha O, et al. Neural Dev. 2023 Oct 13;18(1):7. doi: 10.1186/s13064-023-00175-x. Neural Dev. 2023. PMID: 37833718 Free PMC article. - Evaluation and Validation of Commercially Available Dopamine Transporter Antibodies.
Russo EE, Zovko LE, Nazari R, Steenland H, Ramsey AJ, Salahpour A. Russo EE, et al. eNeuro. 2023 May 4;10(5):ENEURO.0341-22.2023. doi: 10.1523/ENEURO.0341-22.2023. Print 2023 May. eNeuro. 2023. PMID: 37142435 Free PMC article. - Sequences of synaptogenesis in the human fetal and neonatal brain by synaptophysin immunocytochemistry.
Sarnat HB. Sarnat HB. Front Cell Neurosci. 2023 Feb 3;17:1105183. doi: 10.3389/fncel.2023.1105183. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36816854 Free PMC article. Review.
References
- Abitz M, Nielsen RD, Jones EG, Laursen H, Graem N, Pakkenberg B (2007) Excess of neurons in the human newborn mediodorsal thalamus compared with that of the adult. Cereb Cortex 17:2573–2578 - PubMed
- Adickes ED, Folkerth RD, Sims KL (1997) Use of perfusion fixation for improved neuropathologic examination. Arch Pathol Lab Med 121:1199–1206 - PubMed
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW (2007) Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55:687–700 - PubMed
- Andersen BB, Gundersen HJ (1999) Pronounced loss of cell nuclei and anisotropic deformation of thick sections. J Microsc 196:69–73 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous