Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection - PubMed (original) (raw)
Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection
Nicolas Zucchini et al. Int Immunol. 2008 Jan.
Abstract
Plasmacytoid dendritic cells (pDCs) are an important source of IFN-alpha/beta in response to a variety of viruses in vivo, including murine cytomegalovirus (MCMV). However, the respective contributions of various infected organs, and within these of pDCs, conventional dendritic cells and other cells, to the systemic production of IFN-alpha/beta or other innate cytokines during viral infections in vivo is largely unknown. Whether a specialization of pDC subsets in the production of different patterns of innate cytokines exists in vivo in response to a viral infection has not been investigated. Here, by analyzing for the first time directly ex vivo, at the single-cell level, the simultaneous production of up to three cytokines in pDCs isolated from MCMV-infected mice, we show that (i) pDCs are the quasi-exclusive source of IFN-alpha/beta, IL-12 and tumor necrosis factor (TNF)-alpha, early during MCMV infection, in two immunocompetent mouse lines and with two viral strains, (ii) pDC activation for IFN-alpha/beta production is organ specific and (iii) a significant proportion of pDCs simultaneously produce IFN-alpha/beta, TNF-alpha and IL-12, although TNF-alpha and IFN-alpha/beta appear more often co-expressed with one another than each of them with IL-12. Altogether, these results show a broad and non-redundant role of pDCs in early innate detection of, and defense against, viral infection. The data also show differences in the responsiveness of pDCs from different tissues and suggest distinct molecular requirements for pDC production of various cytokines. These observations must be taken into account when designing new antiviral vaccination strategies aimed at harnessing pDC responses.
Figures
Fig. 1.
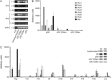
Analysis of the expression of innate cytokine mRNA by DC subsets ex vivo. pDC, CD8α+ cDC and CD8α− cDC were purified from (A) BALB/c or (B and C) C57BL/6 mice, uninfected (0 h) or infected with MCMV for 36 h. (A) RT–PCR analysis of mRNA encoding IFN-α and β in each DC subset purified. (B) GeneChip analysis of various mRNA encoding the Ifna and Ifnb genes in each purified DC subset. (C) GeneChip analysis of various mRNA encoding various innate cytokines and chemokines in each purified leukocyte subset.
Fig. 2.
Evaluation of pDC contribution to innate cytokine production within total splenic leukocytes. C57BL/6 and 129S2 mice were left untreated (0 h) or were infected with the Smith strain of MCMV for different lengths of time (, , or 44 h). (A) The serum levels of IFN-α/β, IL-12p70 and TNF-α were measured by ELISA. (B–E) Expression of the cytokines in combination with the cell-surface markers 120G8 (B, D and E), CD11c (C) or Siglec-H (E) was analyzed by flow cytometry in total splenic leukocytes of 129S2 mice. (B) The percent of cytokine+ cells within the pDC gate (120G8high cells) is indicated on each dot plot. (D) The percent of pDCs (120G8high cells) or of cells of other subtypes (120G8low/int cells) that stained with the anti-IFN-α/β (upper dot plots) or with the isotype control antibodies (lower dot plots) within total splenocytes are indicated on the panel. Results shown are representative of at least two independent experiments with three mice per group. ‘§’ denotes below level of detection.
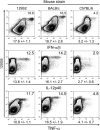
Fig. 3.
Evaluation of pDC contribution to innate cytokine production in response to the infection with the Perth strain of MCMV in 129S2, BALB/c and C57BL/6 mice. 129S2, BALB/c and C57BL6 mice were infected with MCMV Perth for 36 h. Production of IFN-α/β, IL-12 and TNF-α was analyzed by flow cytometry within total splenic leukocytes. The percent of cytokine+ cells within the pDC gate (120G8high cells) is indicated on each dot plot. Results shown are representative of three mice per group.
Fig. 4.
Analysis of different splenic leukocyte populations for IL-12p40 or TNF-α production in C57BL/6 mice. C57BL6 mice were infected with MCMV for 36 h. The expression of IL-12p40 or TNF-α in combination with various cell-surface markers was analyzed by flow cytometry within total splenic leukocytes. (A) Analysis of the co-expression of IL-12p40 or TNF-α with individual cell-surface markers. The number adjacent to each gate corresponds to the percent of the cells from this gate within total splenic leukocytes. (B) Multiparameter analysis of the phenotype of the cytokine+ cells that do not belong to the pDC population. A gate was set on cytokine+120G8−/low/int cells (panel A, upper dot plots) and the co-expression of CD11b and GR-1 was analyzed in this population in comparison to the profile obtained for cytokine+ pDCs (120G8high cells) or for total splenic leukocytes. The number adjacent to the gate corresponds to the percent of GR1lowCD11b+ cells (monocytes/macrophages) within the parent gate.
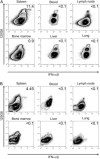
Fig. 5.
Analysis of pDC contribution to IFN-α/β production in different organs. Total leukocytes from different organs of 36 h-infected (A) BALB/c or (B) C57BL/6 mice were analyzed by flow cytometry for the expression of IFN-α/β. Numbers in dot plots represent the percent of cells that produce the cytokine within total pDCs (120G8high). Results shown are representative of two independent experiments with pools of three mice per experiment.
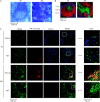
Fig. 6.
Analysis of DC involvement in IFN-α/β production by immunohistofluorescence on spleen. Spleen sections from 129S2 mice infected or not with MCMV were stained with hematoxylin–eosin (A) or with fluorescent-conjugated antibodies (B) and (C). (A and B) Analysis of the structure of the spleen. (C) Localization of pDCs, cDCs and IFN-α/β. Results shown are representative of three experiments each performed on two different infected spleens. Similar results have been obtained with BALB/c mice.
Fig. 7.
Analysis of innate cytokine co-expression within individual pDCs. 129S2 mice were infected with MCMV Smith for 30 or 36 h. Simultaneous analysis of IFN-α/β, IL-12 and TNF-α production gated on pDCs (120G8high). (A) Dot plot analysis. (B and C) Diagrammatic representation of the different populations of pDCs according to their production of innate cytokines after 30 (B) or 36 h (C) of infection with MCMV Smith. Numbers indicate the percentage of cells that produce the corresponding combination of cytokines within pDCs. These results are representative of at least two independent experiments each with three mice per condition. Similar results have been obtained with BALB/c and C57BL/6 mice.
Similar articles
- The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond.
Ngo C, Garrec C, Tomasello E, Dalod M. Ngo C, et al. Cell Mol Immunol. 2024 Sep;21(9):1008-1035. doi: 10.1038/s41423-024-01167-5. Epub 2024 May 22. Cell Mol Immunol. 2024. PMID: 38777879 Free PMC article. Review. - DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulates their function during viral infection.
Sjölin H, Robbins SH, Bessou G, Hidmark A, Tomasello E, Johansson M, Hall H, Charifi F, Karlsson Hedestam GB, Biron CA, Kärre K, Höglund P, Vivier E, Dalod M. Sjölin H, et al. J Immunol. 2006 Sep 1;177(5):2908-16. doi: 10.4049/jimmunol.177.5.2908. J Immunol. 2006. PMID: 16920926 - Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta.
Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dalod M, et al. J Exp Med. 2003 Apr 7;197(7):885-98. doi: 10.1084/jem.20021522. J Exp Med. 2003. PMID: 12682109 Free PMC article. - Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo.
Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Brière F, Trinchieri G, Biron CA. Dalod M, et al. J Exp Med. 2002 Feb 18;195(4):517-28. doi: 10.1084/jem.20011672. J Exp Med. 2002. PMID: 11854364 Free PMC article. - Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat.
Ali S, Mann-Nüttel R, Schulze A, Richter L, Alferink J, Scheu S. Ali S, et al. Front Immunol. 2019 Apr 12;10:778. doi: 10.3389/fimmu.2019.00778. eCollection 2019. Front Immunol. 2019. PMID: 31031767 Free PMC article. Review.
Cited by
- Immune surveillance of cytomegalovirus in tissues.
Mihalić A, Železnjak J, Lisnić B, Jonjić S, Juranić Lisnić V, Brizić I. Mihalić A, et al. Cell Mol Immunol. 2024 Sep;21(9):959-981. doi: 10.1038/s41423-024-01186-2. Epub 2024 Aug 12. Cell Mol Immunol. 2024. PMID: 39134803 Free PMC article. Review. - The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond.
Ngo C, Garrec C, Tomasello E, Dalod M. Ngo C, et al. Cell Mol Immunol. 2024 Sep;21(9):1008-1035. doi: 10.1038/s41423-024-01167-5. Epub 2024 May 22. Cell Mol Immunol. 2024. PMID: 38777879 Free PMC article. Review. - Obesity dysregulates the pulmonary antiviral immune response.
Almond M, Farne HA, Jackson MM, Jha A, Katsoulis O, Pitts O, Tunstall T, Regis E, Dunning J, Byrne AJ, Mallia P, Kon OM, Saunders KA, Simpson KD, Snelgrove RJ, Openshaw PJM, Edwards MR, Barclay WS, Heaney LM, Johnston SL, Singanayagam A. Almond M, et al. Nat Commun. 2023 Oct 19;14(1):6607. doi: 10.1038/s41467-023-42432-x. Nat Commun. 2023. PMID: 37857661 Free PMC article. - M-CSF directs myeloid and NK cell differentiation to protect from CMV after hematopoietic cell transplantation.
Kandalla PK, Subburayalu J, Cocita C, de Laval B, Tomasello E, Iacono J, Nitsche J, Canali MM, Cathou W, Bessou G, Mossadegh-Keller N, Huber C, Mouchiroud G, Bourette RP, Grasset MF, Bornhäuser M, Sarrazin S, Dalod M, Sieweke MH. Kandalla PK, et al. EMBO Mol Med. 2023 Nov 8;15(11):e17694. doi: 10.15252/emmm.202317694. Epub 2023 Aug 28. EMBO Mol Med. 2023. PMID: 37635627 Free PMC article. - Novel mouse models based on intersectional genetics to identify and characterize plasmacytoid dendritic cells.
Valente M, Collinet N, Vu Manh TP, Popoff D, Rahmani K, Naciri K, Bessou G, Rua R, Gil L, Mionnet C, Milpied P, Tomasello E, Dalod M. Valente M, et al. Nat Immunol. 2023 Apr;24(4):714-728. doi: 10.1038/s41590-023-01454-9. Epub 2023 Mar 16. Nat Immunol. 2023. PMID: 36928414 Free PMC article.
References
- Biron CA. Interferons α and β as immune regulators—a new look. Immunity. 2001;14:661. - PubMed
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275. - PubMed
- Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J. Immunol. 2006;177:6263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources