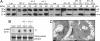The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole - PubMed (original) (raw)
The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole
Eun Ju Sohn et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Plants are unique in their ability to store proteins in specialized protein storage vacuoles (PSVs) within seeds and vegetative tissues. Although plants use PSV proteins during germination, before photosynthesis is fully functional, the roles of PSVs in adult vegetative tissues are not understood. Trafficking pathways to PSVs and lytic vacuoles appear to be distinct. Lytic vacuoles are analogous evolutionarily to yeast and mammalian lysosomes. However, it is unclear whether trafficking to PSVs has any analogy to pathways in yeast or mammals, nor is PSV ultrastructure known in Arabidopsis vegetative tissue. Therefore, alternative approaches are required to identify components of this pathway. Here, we show that an Arabidopsis thaliana mutant that disrupts PSV trafficking identified TERMINAL FLOWER 1 (TFL1), a shoot meristem identity gene. The tfl1-19/mtv5 (for "modified traffic to the vacuole") mutant is specifically defective in trafficking of proteins to the PSV. TFL1 localizes to endomembrane compartments and colocalizes with the putative delta-subunit of the AP-3 adapter complex. Our results suggest a developmental role for the PSV in vegetative tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Floral meristems from wild-type Landsberg erecta, clavata3-2, Vac2, mtv5, and mtv5 complemented with TFL1 driven by its native promoter. (Scale bars, 2 mm.)
Fig. 2.
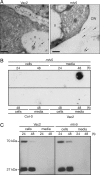
Trafficking defects in mtv5 mutants. (A) CLV3:T7:CTPPBL relocalizes in mtv5. Electron micrographs show that the chimeric protein is trafficked to the vacuole in the Vac2 parental line but trafficked to the apoplasm in mtv5. Arrows designate the locations of gold particles. V, vacuole; CW, cell wall. (Scale bars, 200 nm.) (B) Col-0, Vac2, and mtv5 protoplasts. Protoplasts were incubated for 24 or 48 hours as indicated. Cells and media were separated, and proteins were analyzed in a dot-blot by using an α-T7 antibody. (C) Trafficking of a transiently expressed Arabidopsis aleurain-like protein (AtALP:GFP) is not affected by the mtv5 mutation. The 70-kDa protein is the AALP:GFP fusion, and the 27-kDa protein is GFP that was released from the 70-kDa chimeric protein upon correct delivery to the vacuole. The experiment was carried out as in B, except that protein extracts were resolved using SDS/PAGE and visualized using an α-GFP antibody.
Fig. 3.
Multiple alleles of TFL1 have trafficking defects. (A) Protoplasts were isolated from L_er_, tfl1-2, tfl1-2 complemented with pASM4 (TFL1::TFL1*), tfl1-19, or tfl1-19 complemented with pNVR3068 (TFL1::TFL1). Cells were transformed with GFP:CTPPBL and collected after 24 and 48 hours; the proteins were analyzed by SDS/PAGE and Western blotting by using α-GFP antibodies. (B) (Upper) Dot-blots of media collected after a 48-hour incubation of protoplasts from Vac2, mtv5, and tfl1-1 and tfl1-2 crossed into the Vac2 line. (Lower) Shown is a Coomassie-stained SDS/PAGE gel of the corresponding cell extracts as a loading control. (C) Vacuolar peroxidases are partially relocalized in mtv5. Immunogold-labeling experiments show that Arabidopsis vacuolar peroxidases containing CTPPs are trafficked to the vacuole and cytoplasm in the Vac2 parental line but trafficked to the cell wall and apoplasm in mtv5. Arrows designate the locations of gold particles. V, vacuole; CW, cell wall. (Scale bars, 200 nm.)
Fig. 4.
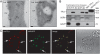
Subcellular localization of TFL1. (A) Immunoelectron microscopy of root tips and shoot meristems demonstrated that TFL1 was localized to the plasma membrane, vacuole, and dense vesicles ≈100 nm in diameter. Localization was identical in mtv5 (data not shown). V, vacuole; CW, cell wall; ve, vesicle; PM, plasma membrane. (Scale bars, 100 nm.) (B) Subcellular distribution of TFL1 protein. Proteins were extracted from protoplasts and centrifuged; the soluble and pellet fractions were analyzed by Western blotting using α-TFL1 antibodies. Anti-VSR1 and anti-aleurain antibodies were used as controls for the membrane and soluble fractions, respectively. Total protein extracts from 2-week-old 35S::TFL1 seedlings and purified recombinant 8XHis:TFL1 from Escherichia coli were used as positive controls for the α-TFL1 antibodies. (C) Protoplasts were transformed with either HA:TFL1 or At_δ_R:GFP constructs. The protoplasts were fixed and visualized with a TRITC-conjugated secondary antibody for HA:TFL1. GFP signals were captured directly from fixed protoplasts. (Scale bar, 20 μm.)
Similar articles
- Genetic interactions reveal the antagonistic roles of FT/TSF and TFL1 in the determination of inflorescence meristem identity in Arabidopsis.
Lee C, Kim SJ, Jin S, Susila H, Youn G, Nasim Z, Alavilli H, Chung KS, Yoo SJ, Ahn JH. Lee C, et al. Plant J. 2019 Aug;99(3):452-464. doi: 10.1111/tpj.14335. Epub 2019 May 17. Plant J. 2019. PMID: 30943325 - Separate elements of the TERMINAL FLOWER 1 cis-regulatory region integrate pathways to control flowering time and shoot meristem identity.
Serrano-Mislata A, Fernández-Nohales P, Doménech MJ, Hanzawa Y, Bradley D, Madueño F. Serrano-Mislata A, et al. Development. 2016 Sep 15;143(18):3315-27. doi: 10.1242/dev.135269. Epub 2016 Jul 6. Development. 2016. PMID: 27385013 - Changing the spatial pattern of TFL1 expression reveals its key role in the shoot meristem in controlling Arabidopsis flowering architecture.
Baumann K, Venail J, Berbel A, Domenech MJ, Money T, Conti L, Hanzawa Y, Madueno F, Bradley D. Baumann K, et al. J Exp Bot. 2015 Aug;66(15):4769-80. doi: 10.1093/jxb/erv247. Epub 2015 May 27. J Exp Bot. 2015. PMID: 26019254 Free PMC article. - The trafficking machinery of lytic and protein storage vacuoles: how much is shared and how much is distinct?
Zhang X, Li H, Lu H, Hwang I. Zhang X, et al. J Exp Bot. 2021 May 4;72(10):3504-3512. doi: 10.1093/jxb/erab067. J Exp Bot. 2021. PMID: 33587748 Review. - Coming into bloom: the specification of floral meristems.
Liu C, Thong Z, Yu H. Liu C, et al. Development. 2009 Oct;136(20):3379-91. doi: 10.1242/dev.033076. Development. 2009. PMID: 19783733 Review.
Cited by
- MTV1 and MTV4 encode plant-specific ENTH and ARF GAP proteins that mediate clathrin-dependent trafficking of vacuolar cargo from the trans-Golgi network.
Sauer M, Delgadillo MO, Zouhar J, Reynolds GD, Pennington JG, Jiang L, Liljegren SJ, Stierhof YD, De Jaeger G, Otegui MS, Bednarek SY, Rojo E. Sauer M, et al. Plant Cell. 2013 Jun;25(6):2217-35. doi: 10.1105/tpc.113.111724. Epub 2013 Jun 14. Plant Cell. 2013. PMID: 23771894 Free PMC article. - FLOWERING LOCUS T/TERMINAL FLOWER1-like genes affect growth rhythm and bud set in Norway spruce.
Karlgren A, Gyllenstrand N, Clapham D, Lagercrantz U. Karlgren A, et al. Plant Physiol. 2013 Oct;163(2):792-803. doi: 10.1104/pp.113.224139. Epub 2013 Aug 19. Plant Physiol. 2013. PMID: 23958861 Free PMC article. - A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in Arabidopsis thaliana.
Lachowiec J, Shen X, Queitsch C, Carlborg Ö. Lachowiec J, et al. PLoS Genet. 2015 Sep 23;11(9):e1005541. doi: 10.1371/journal.pgen.1005541. eCollection 2015. PLoS Genet. 2015. PMID: 26397943 Free PMC article. - Flowering time modulation by a vacuolar SNARE via FLOWERING LOCUS C in Arabidopsis thaliana.
Ebine K, Uemura T, Nakano A, Ueda T. Ebine K, et al. PLoS One. 2012;7(7):e42239. doi: 10.1371/journal.pone.0042239. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848750 Free PMC article. - Genetic inhibition of flowering differs between juvenile and adult Citrus trees.
Muñoz-Fambuena N, Nicolás-Almansa M, Martínez-Fuentes A, Reig C, Iglesias DJ, Primo-Millo E, Mesejo C, Agustí M. Muñoz-Fambuena N, et al. Ann Bot. 2019 Feb 15;123(3):483-490. doi: 10.1093/aob/mcy179. Ann Bot. 2019. PMID: 30289429 Free PMC article.
References
- Paris N, Stanley CM, Jones RL, Rogers JC. Cell. 1996;85:563–572. - PubMed
- Neuhaus JM, Rogers JC. Plant Mol Biol. 1998;38:127–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases