Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans - PubMed (original) (raw)
Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans
Norio Takeshita et al. Mol Biol Cell. 2008 Jan.
Abstract
In filamentous fungi, hyphal extension depends on the continuous delivery of vesicles to the growing tip. Here, we describe the identification of two cell end marker proteins, TeaA and TeaR, in Aspergillus nidulans, corresponding to Tea1 and Mod5 in Schizosaccharomyces pombe. Deletion of teaA or teaR caused zig-zag-growing and meandering hyphae, respectively. The Kelch-repeat protein TeaA, the putatively prenylated TeaR protein, and the formin SepA were highly concentrated in the Spitzenkörper, a vesicle transit station at the tip, and localized along the tip membrane. TeaA localization at tips depended on microtubules, and TeaA was required for microtuble convergence in the hyphal apex. The CENP-E family kinesin KipA was necessary for proper localization of TeaA and TeaR, but not for their transportation. TeaA and TeaR localization were interdependent. TeaA interacted in vivo with TeaR, and TeaA colocalized with SepA. Sterol-rich membrane domains localized at the tip in teaA and teaR mutants like in wild type, and filipin treatment caused mislocalization of both proteins. This suggests that sterol-rich membrane domains determine cell end factor destinations and thereby polarized growth.
Figures
Figure 1.
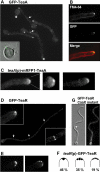
Characterization of TeaA and TeaR. (A) Scheme of A. nidulans TeaA, S. pombe Tea1, and S. cerevisiae Kel1. All three proteins share Kelch-repeats (gray boxes) at the N terminus and extended coiled-coil regions (black boxes) in the C-terminal half of the proteins. (B) Scheme of A. nidulans TeaR and S. pombe Mod5. The proteins are characterized by a C-terminal CAAX prenylation motif.
Figure 2.
Phenotypic comparison of Δ_kipA_, Δ_teaA_, Δ_teaR_, and corresponding double mutants. (A) Colonies of wild type (GR5), Δ_kipA_ (SRL1), Δ_teaA_ (SSK91), Δ_teaR_ (SNT33), Δ_kipA/Δ_teaA (SNT14), Δ_kipA/Δ_teaR (SNT40), and Δ_teaA/Δ_teaR (SNT39) strains. Strains were grown on minimal medium glucose agar plates for 2 d. (B) Differential interference contrast images of wild type, Δ_kipA_, Δ_teaA_, Δ_teaR_, Δ_kipA/Δ_teaA, Δ_kipA/Δ_teaR, and Δ_teaA/Δ_teaR strains as indicated. Strains were grown on microscope slides coated with minimal medium, with glucose and 0.8% agarose for 1 d (top and bottom). Strains were grown on minimal medium glucose agar plates for 2 d (middle). Hyphae are 3–4 μm in diameter. (C) Quantification of the effect of the gene deletions on second germ tube formation. Conidia of each strain were germinated in minimal medium with glucose, and then they were analyzed for the emergence of the second germ tube. For each strain, 200 germlings were counted.
Figure 3.
TeaA and TeaR localization. Strains SNT4 (A and B), SNT49 (C), SNT26 (D, E), SNT55 (F), and SNT61 (G) were grown on minimal medium with glycerol as carbon source. (A) GFP-TeaA localized to one point in the apex of all hyphal tips and some points in the cell body (arrows). The TeaA spot showed up in conidia before germination (left bottom). (B) The membrane was stained with FM4-64 (top, red in merged image). The Spitzenkörper labeled by FM4-64 colocalized with GFP-TeaA point (middle, green in merged image) at the tip. (C) mRFP1-TeaA produced under native promoter control localized to one point at most of tips and weaker signals were observed along the tip membrane. (D) GFP-TeaR localized to one point at hyphal tips and along the apex (left inset) and septa (arrows, right inset). (E) GFP-TeaR localized to one main point and two smaller points next to the main point at hyphal tips (left). GFP-TeaR spots aligned along the apex (right). (F) The percentage of GFP-TeaR localization pattern; 100 hyphal tips were analyzed. Hyphae are 3–4 μm in diameter.
Figure 4.
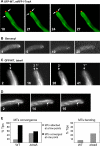
Relationship between TeaA and microtubules (MT). (A) In strain SNT22 (GFP-MT, mRFP1-TeaA), GFP-MTs elongated toward the hyphal tip (arrow) and merged at one point at the apex. The point colocalized with mRFP1-TeaA point at the apex (see Supplemental Movie 1). Times are indicated in seconds. (B) SNT49 [teaA(p)-mRFP1-TeaA] was grown in minimal medium with glycerol for 1 d and treated with the medium containing 2.5 μg/ml benomyl. mRFP1-TeaA point at tips was sometimes divided into a few points and disappeared from >80% of tips after 30 min (see Table 3). Elapsed time is given in minutes. (C) In strain SNT 31 (Δ_teaA_, GFP-MT), GFP-MTs did not merge in one point at the apex but instead attached to a few points (arrows indicate points where MT attached; see Supplemental Movie 2). Elapsed time is given in seconds. (D) In the strain SNT 31, GFP-MTs became more curved than those in wild type. GFP-MTs bent, probably because they kept growing after they reached the cortex (arrows; see Supplemental Movie 3). Elapsed time is given in seconds. Hyphae are 3–4 μm in diameter. (E) Quantification of MTs behavior in SNT22 (GFP-MT, mRFP1-TeaA) and SNT31 (Δ_teaA_, GFP-MT). The percentage of tips where GFP-MTs merged in one point (black bar) or attached to a few points (gray bar) (left), and the percentage of tips where GFP-MTs bent after they attached to the cortex (right). Data from 100 tips of strain SNT22 and SNT31 during 1-min observation.
Figure 5.
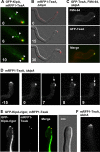
Relationship between TeaA and KipA. (A) In strain SNT23 (GFP-KipA, mRFP1-TeaA), GFP-KipA spots, which label MT plus ends, moved toward the hyphal tip (arrows), and they merged at one point at the apex with mRFP1-TeaA (see Supplemental Movie 4). Elapsed time is given in seconds. (B and C) In the Δ_kipA_ mutant (SNT41), mRFP1-TeaA still localized to one point at the hyphal tip but often moved away from the center of the apex (see Table 3). Elapsed time is given in minutes. (C) In the Δ_kipA_ mutant (SNT9), the Spitzenkörper labeled by FM4-64 (top, red in merged image) also often moved away from the center of the apex and colocalized with the GFP-TeaA point at the tips (middle, green in merged image). (D) In the Δ_kipA_ mutant (SNT50), mRFP1-TeaA moved away from the center of the apex to the side of the tip, and it divided into two points (arrows; see Supplemental Movie 5). Elapsed time is given in minutes. (E) In the _kipA_-rigor mutant (SNT51), mRFP1-TeaA sometimes localized to two points at the tip (see Table 3), whereas GFP-KipAG223E decorated MTs and MTs attached to the two points. (F) Deletion of kinA did not affect mRFP1-TeaA tip localization (strain SNT62). Hyphae are 3–4 μm in diameter.
Figure 6.
Interaction of TeaA, SepA and TeaA, TeaR. (A) GFP-SepA and mRFP1-TeaA colocalized to one point at the apex and along the apex in strain SNT57. (B) Strain SNT57 grown in minimal medium with glycerol for 1 d was treated with the medium containing 2 μg/ml cytochalasin A for 30 min. (C) In strain SNT56, the mRFP1-TeaA point at the apex colocalized with that of GFP-TeaR, although their localization was not identical. (D) BiFC analysis of TeaA and TeaR. In SNT59 expressing TeaR tagged with the N-terminal half of YFP and TeaA tagged with the C-terminal half of YFP, the YFP signal was detected at the tip. (E) Yeast two-hybrid interaction between the DNA binding domain fused to the TeaA N-terminal half (BD-TeaA,N) and the activation domain fused to the TeaA N- or C-terminal half (AD-TeaA,N, AD-TeaA,C), TeaR full length (AD-TeaR), or as control the empty vector (AD). The mated yeasts were selected on SD/−Leu/−Trp plate (top) and grown on nutritionally selective plate SD/−Leu/−Trp/-His (bottom). β-Galactosidase activity was analyzed by liquid culture assay using ONPG as substrate. The value of BD-TeaA,N and AD was used as a standard (100%). The data are expressed as the mean ± SD (n = 4).
Figure 7.
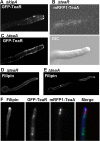
Localization dependency of KipA, TeaA, TeaR, and sterol-rich regions. (A) In the Δ_kipA_ mutant (SNT43), some GFP-TeaR signal localized at the membrane of the apex, and other signal dispersed along the membrane away from the tip. (B) In the Δ_teaR_ mutant (SNT53), mRFP1-TeaA was not observed at the tip. (C) In the Δ_teaA_ mutant (SNT32), GFP-TeaR lost the preference for the hyphal tip and diffused all along the membrane. (D and E) The Δ_teaR_ (SNT33) and Δ_teaA_ (SSK91) mutants were stained with 10 μg/ml filipin for 5 min. (F) Strain SNT27 was treated with 10 μg/ml filipin for 5 min. Filipin accumulated at the tip (blue in merged image), GFP-TeaR lost the membrane association (green in merged image), and mRFP1-TeaA dispersed at the tip (red in merged image). Hyphae are 3–4 μm in diameter.
Figure 8.
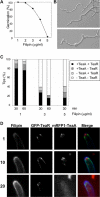
Effect of filipin treatment on hyphal growth and localization of TeaA and TeaR. (A) Germination efficiency. Conidia of wild type (GR5) were inoculated in minimal medium with glycerol in the presence of 1–5 μg/ml filipin, incubated for 36 h, and analyzed for germ tube emergence. Two hundred germlings or conidia were counted. (B) Differential interference contrast images of wild type grown in minimal medium with glycerol and 3 μg/ml filipin for 36 h. (C) Quantification of the number of tips where mRFP1-TeaA and GFP-TeaR localized correctly or not at the tip membrane. Strain SNT56 grown in minimal medium with glycerol for 24 h was treated with the medium containing 1, 3, or 5 μg/ml filipin for 30 or 60 min. Percentage of the tips with the correct localization of mRFP1-TeaA and GFP-TeaR (black bar), with only mRFP1-TeaA (dark gray bar), with only GFP-TeaR (bright gray bar), and without both (white bar); 100 tips were counted. (D) Time course illustration of the localization patterns after the treatment with 3 μg/ml filipin quantified in C. Elapsed time is given in minutes. Hyphae are 3–4 μm in diameter.
Similar articles
- The cell end marker protein TeaC is involved in growth directionality and septation in Aspergillus nidulans.
Higashitsuji Y, Herrero S, Takeshita N, Fischer R. Higashitsuji Y, et al. Eukaryot Cell. 2009 Jul;8(7):957-67. doi: 10.1128/EC.00251-08. Epub 2009 May 8. Eukaryot Cell. 2009. PMID: 19429780 Free PMC article. - On the role of microtubules, cell end markers, and septal microtubule organizing centres on site selection for polar growth in Aspergillus nidulans.
Takeshita N, Fischer R. Takeshita N, et al. Fungal Biol. 2011 Jun;115(6):506-17. doi: 10.1016/j.funbio.2011.02.009. Epub 2011 Feb 19. Fungal Biol. 2011. PMID: 21640315 - The cell-end marker TeaA and the microtubule polymerase AlpA contribute to microtubule guidance at the hyphal tip cortex of Aspergillus nidulans to provide polarity maintenance.
Takeshita N, Mania D, Herrero S, Ishitsuka Y, Nienhaus GU, Podolski M, Howard J, Fischer R. Takeshita N, et al. J Cell Sci. 2013 Dec 1;126(Pt 23):5400-11. doi: 10.1242/jcs.129841. Epub 2013 Oct 7. J Cell Sci. 2013. PMID: 24101725 - Polarized growth in fungi--interplay between the cytoskeleton, positional markers and membrane domains.
Fischer R, Zekert N, Takeshita N. Fischer R, et al. Mol Microbiol. 2008 May;68(4):813-26. doi: 10.1111/j.1365-2958.2008.06193.x. Epub 2008 Apr 8. Mol Microbiol. 2008. PMID: 18399939 Review. - Where to grow and where to go.
Kriegler M, Herrero S, Fischer R. Kriegler M, et al. Fungal Genet Biol. 2025 May;178:103983. doi: 10.1016/j.fgb.2025.103983. Epub 2025 Apr 3. Fungal Genet Biol. 2025. PMID: 40187481 Review.
Cited by
- Holophytochrome-Interacting Proteins in Physcomitrella: Putative Actors in Phytochrome Cytoplasmic Signaling.
Ermert AL, Mailliet K, Hughes J. Ermert AL, et al. Front Plant Sci. 2016 May 12;7:613. doi: 10.3389/fpls.2016.00613. eCollection 2016. Front Plant Sci. 2016. PMID: 27242820 Free PMC article. - Fungal sphingolipids: role in the regulation of virulence and potential as targets for future antifungal therapies.
Mota Fernandes C, Del Poeta M. Mota Fernandes C, et al. Expert Rev Anti Infect Ther. 2020 Nov;18(11):1083-1092. doi: 10.1080/14787210.2020.1792288. Epub 2020 Jul 16. Expert Rev Anti Infect Ther. 2020. PMID: 32673125 Free PMC article. Review. - Reorganization of the growth pattern of Schizosaccharomyces pombe in invasive filament formation.
Dodgson J, Brown W, Rosa CA, Armstrong J. Dodgson J, et al. Eukaryot Cell. 2010 Nov;9(11):1788-97. doi: 10.1128/EC.00084-10. Epub 2010 Sep 24. Eukaryot Cell. 2010. PMID: 20870879 Free PMC article. - Dynamics of Actin Cables in Polarized Growth of the Filamentous Fungus Aspergillus nidulans.
Bergs A, Ishitsuka Y, Evangelinos M, Nienhaus GU, Takeshita N. Bergs A, et al. Front Microbiol. 2016 May 9;7:682. doi: 10.3389/fmicb.2016.00682. eCollection 2016. Front Microbiol. 2016. PMID: 27242709 Free PMC article. - Microtubule Motors in Establishment of Epithelial Cell Polarity.
Kreitzer G, Myat MM. Kreitzer G, et al. Cold Spring Harb Perspect Biol. 2018 Feb 1;10(2):a027896. doi: 10.1101/cshperspect.a027896. Cold Spring Harb Perspect Biol. 2018. PMID: 28264820 Free PMC article. Review.
References
- Browning H., Hackney D. D., Nurse P. Targeted movement of cell end factors in fission yeast. Nat. Cell Biol. 2003;5:812–818. - PubMed
- Busch K. E., Hayles J., Nurse P., Brunner D. Tea2p kinesin is involved in spatial microtubule organization by transporting tip1p on microtubules. Dev. Cell. 2004;16:831–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous