Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish - PubMed (original) (raw)
Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish
Hilary Clay et al. Cell Host Microbe. 2007.
Abstract
In tuberculosis, infecting mycobacteria are phagocytosed by macrophages, which then migrate into deeper tissue and recruit additional cells to form the granulomas that eventually contain infection. Mycobacteria are exquisitely adapted macrophage pathogens, and observations in the mouse model of tuberculosis have suggested that mycobacterial growth is not inhibited in macrophages until adaptive immunity is induced. Using the optically transparent and genetically tractable zebrafish embryo-Mycobacterium marinum model of tuberculosis, we have directly examined early infection in the presence and absence of macrophages. The absence of macrophages led rapidly to higher bacterial burdens, suggesting that macrophages control infection early and are not an optimal growth niche. However, we show that macrophages play a critical role in tissue dissemination of mycobacteria. We propose that residence within macrophages represents an evolutionary trade-off for pathogenic mycobacteria that slows their early growth but provides a mechanism for tissue dissemination.
Figures
Fig 1
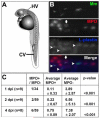
Macrophages are the primary cell to phagocytose M. marinum. A) Diagram of one day post fertilization (dpf) zebrafish embryo showing caudal vein (CV) and hindbrain ventricle (HV) injection sites. B) Dual fluorescent labeling of myeloperoxidase (MPO) and L-plastin antibodies in two dpf fish infected with green fluorescent M. marinum (Mm). Infected macrophages as indicated by L-plastin-positive, MPO-negative staining are indicated with arrowheads. An MPO-positive cell that is partially colocalized with GFP expressing bacteria is either adjacent to or possibly phagocytosing a bacterium is indicated with an arrow. Images are taken from the caudal vein. Scale bar, 25 μm. C) Numbers of infected MPO-positive cells versus all infected phagocytes in a low-dose infection (12 ± 3 CFU) analyzed at two, three, and five dpi. Data are presented as total number of infected MPO-positive cells out of total number of infected _M. marinum_- containing cells for all fish, and as average number of MPO-positive and negative cells per fish ± the standard deviation. Statistics were calculated using Student′s paired t-test for average numbers of MPO-positive versus MPO-negative cells per fish.
Fig 2
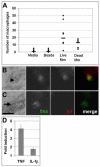
Macrophages undergo rapid functional and molecular changes in response to mycobacterial infection. A**)** Graph of number of macrophages recruited six hours after HV injection. Mm is M. marinum. Medians are indicated by bars. Data from each condition were compared using a Kruskall-Wallis non-parametric ANOVA (p<0.0001). p<0.01 for all pair wise comparisons of medium or beads vs. live or dead bacteria. Difference in median number of macrophages recruited by live vs. dead bacteria was not significant. B, C) Differential interference contrast (DIC) (left) and fluorescence images of five dpf infected (B) and uninfected (C) embryos stained using double fluorescence in situ hybridization for c-fms (green) and tnf (red). Macrophages imaged here were in the caudal vein. Arrowhead in B indicates bacteria within a macrophage visible by DIC microscopy; arrow in C indicates position of uninfected macrophage. Scale bar, 10 μm. D) Quantitative real time PCR values for whole fish one dpi plotted as fold increase over mock injection for TNF at a low dose infection (32 ± 4 CFU, n = 3, p<0.05 using a one sample t-test against a hypothetical mean of 1.0) and IL-1β at a high dose infection (205 ± 38 CFU, n = 4, p<0.05). Error bars represent standard deviation.
Fig 3
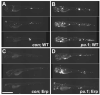
Mycobacteria achieve higher burdens in pu.1 morphant embryos lacking macrophages. Control (A) and pu.1 morphants (B) infected with map49::gfp;msp12::dsRed bacteria that are constitutively red fluorescent and both red and green fluorescent upon macrophage infection, shown here at four dpi. Scale bar, 50 μm. C) Mean bacterial colony forming units (CFUs) per embryo at two and four dpi. Error bars represent standard deviations. Mean CFUs from in control vs. pu.1 morphants were significantly different at both time points (p<0.01 at two dpi and four dpi, p<0.05 at four dpi HV using Student′s unpaired t-test). Inoculum for caudal vein infections was 35 ± 8 CFU, n=5 sets of four fish for each data set. Inoculum for hindbrain ventricle (HV) infections was 14 ± 5, n=5 sets of four control and n=4 sets of three pu.1 morphants.
Fig 4
The absence of macrophages rescues the growth defect of the M. marinum Erp-deficient mutant. For each condition, ten embryos were infected and monitored for four dpi. Images were obtained under the same settings for all embryos and assembled for each condition in descending order of fluorescence (indicating infectivity) as judged visually. Very little difference was noted within embryos in a given condition. The three most infected individual embryos are shown for each condition. Four dpi control (A, C) and pu.1 morphant (B, D) embryos infected with 50 ± 16 CFU of wild-type (A, B) or 56 ± 6 Erp-deficient M. marinum (C, D). Fluorescence represents infecting bacteria in all panels. Scale bar, 250 μm.
Fig 5
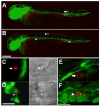
Infecting mycobacteria fail to disseminate to tissues in the absence of macrophages. A-C) Control and pu.1 morphants injected at 22 hpf into the hindbrain ventricle and scored for dissemination out of the ventricle at 16 hpi. Macrophages are visible in the ventricle of a control (A) embryo, an infected macrophage is indicated by an arrow. A pu.1 morphant (B) has extracellular bacteria (arrowhead) in the hindbrain with no evidence of macrophages in the cavity. Fluorescence is slightly blurred due to Brownian motion of unanchored bacteria in the cavity. C) An infected control macrophage has disseminated out of the hindbrain ventricle and is shown here in the trunk of the tail. Scale bars, 50 μm (A, B), 25 μm (C).
Fig 6
Infecting mycobacteria remain in the vasculature in the absence of macrophages. fli1:EGFP transgenic embryos infected with 161 ± 10 red fluorescent bacteria imaged 16 hpi by widefield (A-D) or confocal microscopy (E, F). Whole fish overlays of green fluorescent vasculature and red fluorescent injected bacteria for control (A) and pu.1 morphants (B). Yolk and yolk extension appear red due to autofluorescence. Boxes in (A) and (B) are the areas shown magnified in (C) and (D), respectively. Arrows in control embryos show bacteria that do not colocalize with green fluorescent vasculature indicating they have migrated into tissue (A, C, E). DIC imaging in the right half of panel f shows that bacteria are within a macrophage. Arrowheads in pu.1 morphant shows a bacterium colocalizing with the vasculature indicating it has not migrated into tissues (B, D, F). DIC imaging in the right half of panel (D) shows that the bacterium is not in a macrophage. Three dimensional reconstruction images (maximum intensity) of 48 hpi control (E) and pu.1 morphant embryos (F). Movies of rotational views of these images are provided in supplemental movies 1 and 2 to show in greater detail the spatial relationship of the bacteria to the vasculature. Scale bars, 250 μm (A, B), 25 μm (C, D), 15μm (E, F).
Comment in
- The complex relationship between mycobacteria and macrophages: it's not all bliss.
Fortune SM, Rubin EJ. Fortune SM, et al. Cell Host Microbe. 2007 Jul 12;2(1):5-6. doi: 10.1016/j.chom.2007.06.008. Cell Host Microbe. 2007. PMID: 18005712
Similar articles
- The complex relationship between mycobacteria and macrophages: it's not all bliss.
Fortune SM, Rubin EJ. Fortune SM, et al. Cell Host Microbe. 2007 Jul 12;2(1):5-6. doi: 10.1016/j.chom.2007.06.008. Cell Host Microbe. 2007. PMID: 18005712 - Infection and RNA-seq analysis of a zebrafish tlr2 mutant shows a broad function of this toll-like receptor in transcriptional and metabolic control and defense to Mycobacterium marinum infection.
Hu W, Yang S, Shimada Y, Münch M, Marín-Juez R, Meijer AH, Spaink HP. Hu W, et al. BMC Genomics. 2019 Nov 20;20(1):878. doi: 10.1186/s12864-019-6265-1. BMC Genomics. 2019. PMID: 31747871 Free PMC article. - Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity.
Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. Swaim LE, et al. Infect Immun. 2006 Nov;74(11):6108-17. doi: 10.1128/IAI.00887-06. Infect Immun. 2006. PMID: 17057088 Free PMC article. - Looking within the zebrafish to understand the tuberculous granuloma.
Ramakrishnan L. Ramakrishnan L. Adv Exp Med Biol. 2013;783:251-66. doi: 10.1007/978-1-4614-6111-1_13. Adv Exp Med Biol. 2013. PMID: 23468113 Review. - Transcriptomic Approaches in the Zebrafish Model for Tuberculosis-Insights Into Host- and Pathogen-specific Determinants of the Innate Immune Response.
Benard EL, Rougeot J, Racz PI, Spaink HP, Meijer AH. Benard EL, et al. Adv Genet. 2016;95:217-51. doi: 10.1016/bs.adgen.2016.04.004. Epub 2016 Jun 14. Adv Genet. 2016. PMID: 27503359 Review.
Cited by
- Think small: zebrafish as a model system of human pathology.
Goldsmith JR, Jobin C. Goldsmith JR, et al. J Biomed Biotechnol. 2012;2012:817341. doi: 10.1155/2012/817341. Epub 2012 Jun 3. J Biomed Biotechnol. 2012. PMID: 22701308 Free PMC article. Review. - IL-10 Impairs Local Immune Response in Lung Granulomas and Lymph Nodes during Early Mycobacterium tuberculosis Infection.
Wong EA, Evans S, Kraus CR, Engelman KD, Maiello P, Flores WJ, Cadena AM, Klein E, Thomas K, White AG, Causgrove C, Stein B, Tomko J, Mattila JT, Gideon H, Lin PL, Reimann KA, Kirschner DE, Flynn JL. Wong EA, et al. J Immunol. 2020 Feb 1;204(3):644-659. doi: 10.4049/jimmunol.1901211. Epub 2019 Dec 20. J Immunol. 2020. PMID: 31862711 Free PMC article. - Zebrafish: an underutilized tool for discovery in host-microbe interactions.
Stream A, Madigan CA. Stream A, et al. Trends Immunol. 2022 Jun;43(6):426-437. doi: 10.1016/j.it.2022.03.011. Epub 2022 May 5. Trends Immunol. 2022. PMID: 35527182 Free PMC article. Review. - RNAseq Profiling of Leukocyte Populations in Zebrafish Larvae Reveals a cxcl11 Chemokine Gene as a Marker of Macrophage Polarization During Mycobacterial Infection.
Rougeot J, Torraca V, Zakrzewska A, Kanwal Z, Jansen HJ, Sommer F, Spaink HP, Meijer AH. Rougeot J, et al. Front Immunol. 2019 Apr 17;10:832. doi: 10.3389/fimmu.2019.00832. eCollection 2019. Front Immunol. 2019. PMID: 31110502 Free PMC article. - Host-pathogen interactions made transparent with the zebrafish model.
Meijer AH, Spaink HP. Meijer AH, et al. Curr Drug Targets. 2011 Jun;12(7):1000-17. doi: 10.2174/138945011795677809. Curr Drug Targets. 2011. PMID: 21366518 Free PMC article. Review.
References
- Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. - PubMed
- Alsaadi AI, Smith DW. The fate of virulent and attenuated Mycobacteria in guinea pigs infected by the respiratory route. Am Rev Respir Dis. 1973;107:1041–1046. - PubMed
- Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, Hill AV. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–724. - PubMed
- Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–644. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 GM07270/GM/NIGMS NIH HHS/United States
- R01 AI54503/AI/NIAID NIH HHS/United States
- T32 GM007270/GM/NIGMS NIH HHS/United States
- K22 F007309/PHS HHS/United States
- R01 GM074827/GM/NIGMS NIH HHS/United States
- R01 AI036396/AI/NIAID NIH HHS/United States
- R01 AI054503/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases