Identification of human MVB12 proteins as ESCRT-I subunits that function in HIV budding - PubMed (original) (raw)
Identification of human MVB12 proteins as ESCRT-I subunits that function in HIV budding
Eiji Morita et al. Cell Host Microbe. 2007.
Abstract
Human ESCRT-I is a multiprotein complex that plays essential roles in HIV budding and endosomal protein sorting. All ESCRT-I complexes contain three common subunits (TSG101, VPS28, and VPS37), and a fourth subunit of yeast ESCRT-I was recently identified (Mvb12p). We now demonstrate that two related human proteins (MVB12A and MVB12B) constitute the fourth class of metazoan ESCRT-I subunits, despite lacking identifiable sequence homology to Mvb12p. Hydrodynamic studies indicate that soluble human ESCRT-I complexes contain one copy of each of the four subunit types. MVB12 subunits associate with the core region of the binary TSG101-VPS37 complex through conserved C-terminal sequence elements. Both MVB12 depletion and overexpression inhibit HIV-1 infectivity and induce unusual viral assembly defects, including aberrant virion morphologies and altered viral Gag protein processing. Taken together, these studies define the composition of human ESCRT-I complexes and indicate that the MVB12 subunits play a unique role in regulating ESCRT-mediated virus budding.
Figures
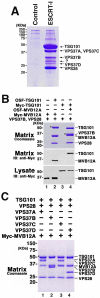
Fig. 1. Subunit composition of human ESCRT-I complexes
(A) Affinity purification and analysis of Strep-tagged TSG101/ESCRT-I Complexes. All six previously known human ESCRT-I subunits (One-STrEP-FLAG-(OSF)-TSG101, VPS28, VPS37A-D) were simultaneously co-expressed in 293T cells. The resulting complexes were affinity purified on a Strep-Tactin matrix, and visualized by SDS-PAGE (lane 2, Coomassie staining). ESCRT-I subunits co-affinity purifying with OSF-TSG101 are labeled at right, and the band labeled “?” was identified as MVB12A (NCBI: NP_612410). Lane 1 shows background protein binding levels from a control purification from cells transfected with an empty vector. (B) MVB12A forms a stable, stoichiometric complex with TSG101, VPS28, and VPS37B. Lanes 2 and 4 show affinity purification of the quaternary complex when the four proteins were co-expressed with OSF-TSG101 (lane 2) or OSF-MVB12A (lane 4). Asterisks here and throughout denote the tagged “bait” protein. Lanes 1 and 3 show control experiments with extracts that lacked the OSF-tagged subunit. Upper panel: SDS-PAGE gel showing all co-purifying proteins (Coomassie staining). Lower panels: Western blot (anti-Myc) detection of Myc-MVB12A (lanes 1, 2) or Myc-TSG101 (lanes 3, 4) bound to the Strep-Tactin matrix (middle panel) or in the input lysate (lower panel). Note that MVB12A migration changes with the presence or absence of the affinity tag. (C) MVB12A forms stable, stoichiometric ESCRT-I complexes with all four known VPS37 subunits. SDS-PAGE gel (Coomassie staining) showing the proteins that co-affinity purify with OSF-TSG101 from lysates containing overexpressed OSF-TSG101, VPS28, MVB12A, and one of the following: Vps37A (lane 1), Vps37B (lane 2), Vps37C (lane 3), Vps37D (lane 4).
Fig. 2. Mapping MVB12-ESCRT-I Interactions
(A) Deletion analysis showing the minimal ESCRT-I binding regions on MVB12A and MVB12B. Upper panel shows binding of the TSG101-VPS28-VPS37B ESCRT-I ternary complex to a deletion series of OSF-MVB12A (lanes 2-7) and OSF-MVB12B (Lanes 9-11). Lanes 1 and 8 show background ESCRT-I binding in the absence of the OSF-MVB12 subunit (negative controls). Proteins were visualized by Coomassie staining, and OSF-MVB12 “baits” are designated with asterisks. The lower panel shows a western blot of the input lysates (anti-Myc), confirming the presence of Myc-TSG101. (B) MVB12A binds binary TSG101-VPS37 complexes. Co-purification of Myc-MVB12A with different OSF-tagged and untagged ESCRT-I subunits (indicated above). Upper panel: western blots testing Myc-MVB12A binding to different ESCRT-I subunits and subcomplexes. Lower and middle panels: control western blots confirming expression of MVB12A (middle) and OSF-tagged protein binding to the Strep-Tactin matrix (lower). Controls show lack of Myc-MVB12A binding in the absence of other ESCRT-I subunits (negative control, lane 1), and Myc-MVB12A binding to the TSG101-VPS28-VPS37 ternary complex (positive control, lane 12). (C) Deletion analysis mapping of the minimal MVB12A binding sites on TSG101 and VPS37B. SDS-PAGE gel (Coomassie staining) showing co-purification of full length Myc-MVB12A with different OSF-tagged TSG101 and VPS37B constructs. OSF-tagged baits are given above and are designated by asterisks. Lower panel: Control western blot of the input lysates, confirming the presence of Myc-MVB12A. Middle panel: western blot of the purified complexes (anti-Myc), confirming the presence or absence of Myc-MVB12A. (D) Summary of the ESCRT-I core interaction sites for TSG101, VPS28, VPS37, and MVB12. Core binding regions of TSG101, VPS28, VPS37(A) and MVB12(A) are shown in lighter shades.
Fig. 3. Gel Filtration Chromatography of ESCRT-I Complexes
Panels 1 and 2: Gel filtration mobility and homogeneity of endogenous ESCRT-I complexes, as detected by western blotting of crude K562 cell lysates fractionated by gel filtration chromatography and detected with an anti-TSG101 antibody (Panel 1, apparent MW ∼270 KDa) or an anti-MVB12A antibody. (Panel 2, apparent MW ∼280 KDa). Assayed fractions are given above, together with the positions of molecular weight size markers. Detection with anti-VPS28 and anti-VPS37B antibodies yielded similar results (not shown). Note that breaks between lanes reflect samples run on two different gels. Panels 3-5: Gel filtration mobilities and homogeneity of overexpressed ESCRT-I complexes from 293T cells. Panel 3: Overexpressed OSF-TSG101-VPS28-VPS37B ternary complexes (anti-FLAG detection, MWapp ∼210 KDa), Panel 4: OSF-TSG101-VPS28-VPS37B-Myc-MVB12A quaternary complexes (anti-Myc detection, MWapp ∼270). Panel 5: OSF-TSG101-VPS28-VPS37B-Myc-MVB12B quaternary complexes (anti-Myc detection, MWapp ∼270 KDa).
Fig. 4. Characterization of Pure Recombinant ESCRT-I Complexes
(A) Purified recombinant ESCRT-I binds HIV-1 p6Gag and HRS. Upper Panels: western blots detecting OSF-TSG101 (anti-Flag) or MVB12A (anti-MVB12A). Left Panel: 2% of input ESCRT-I, Central Panel: ESCRT-I binding to GST (negative control), wt HIV-1 p6Gag, or HIV-1 p6Gag P10A mutant, Right Panel: ESCRT-I binding to V5-HRS or to a mock purified control. Lower panels: SDS-PAGE (Coomassie staining) showing purified ESCRT-I (left panel, 20% input), GST and GST-p6Gag fusion proteins (middle panel), and V5-HRS (right panel). (B) The solution mass of the major complex of purified recombinant human ESCRT-I corresponds to a monomeric 1:1:1:1 complex of OSF-TSG101:VPS27:VPS28:MVB12A. The equilibrium sedimentation distribution of purified ESCRT-I complexes is shown (16,000 rpm, 3.6 μM ESCRT-I, 4°C). The black line shows the global fit of an ideal single species model to centrifugation data from two speeds and two protein concentrations (MWest=130 KDa, MWmonomer=134 KDa). For comparison, the distribution expected for a 2:2:2:2 dimeric ESCRT-I complex is shown in red.
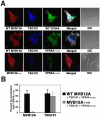
Fig. 5. Mutant VPS4 Proteins Relocalize TSG101 and MVB12A to Class E MVB Compartments
(A) Confocal immunofluorescence z-slices showing co-localization of different OSF-MVB12A (red), Myc-TSG101 (blue) and VPS4A-GFP (green) proteins (note that untagged VPS28 and VPS37B were also co-overexpressed). Top Row: Co-localization of the wild type proteins, Middle Row: Co-localization with a VPS4A ATPase binding mutant (VPS4K173Q), Lower Row: Co-localization with VPS4AK173Q, and a truncated MVB12A protein (MVB12A1-191) that lacked the ESCRT-I binding site. Merged images are shown in column 4, with white indicating full co-localization, and DIC images are shown in column 5. Scale bars represent 10 μM (B) Quantification of the percentage co-localization of VPS4AK173Q with MVB12A or TSG101 in the presence of WT MVB12A or MVB12A1-191 (n= 20 cells/data point).
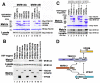
Fig. 6. siRNA Depletion of MVB12 Proteins Inhibits HIV-1 Infectivity and Viral Maturation
(A) siRNA depletion of endogenous MVB12A (upper panel) and overexpressed MVB12B (lower panel). siRNA names denote the first nucleotide targeted by the 21 nt (19 nt + TT overhang) siRNA duplexes. (B) Upper panel: reduction in HIV-1 infectivity upon depletion of MVB12 proteins. Middle panel: viral particle release as analyzed by western blotting. Lower panel: cellular MA and CA expression levels. (C) EM analysis and quantification of viral maturation phenotypes from control cells (white bars) or from cells depleted of MVB12A (grey) or MVB12B (black). EM images show representative examples of Mature and Condensed phenotypes, together with a continuum of Amorphous phenotypes. Percentages were derived by scoring >40 randomly selected virions released under each condition. Note that “Condensed” phenotypes may simply arise when mature conical cores are imaged in cross section. Scale bars are 10 nm.
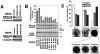
Fig. 7. MVB12 Overexpression Inhibits HIV-1 Release, Infectivity, and Processing
(A) Reductions in HIV-1 release and infectivity upon overexpression of MVB12A or MVB12B. Left panel shows infectivity data. Upper right panel is a western blot showing levels of HIV-1 particles released into the supernatant (anti-MA and anti-CA detection). Middle right panel shows cellular expression levels and proteolytic processing of Gag (anti-MA and anti-CA detection). Lower right panel shows cellular MVB12 expression levels (anti-Myc). Western blotting data from lane 2 in this figure and in Fig. 7D were quantified to determine changes in protein levels upon MVB12A overexpression (relative to control conditions, lanes 1): Cell-associated Gag protein, 71±8%; Cell-associated CA+MA proteins, 11±3%; Virion-associated CA+MA proteins, 16±5%. (B) EM analyses showing aberrant particles released from 293T cells depleted of MVB12A (upper panel) or TSG101 lower panel. (C) Summary of the mapped (black) or putative (white) phosphorylation sites on MVB12A and MVB12B (upper two panels) and MVB12A 5A and 9A mutant constructs (lower two panels). (D) MVB12A phosphorylation mutations abrogate dominant inhibition of HIV-1 release and infectivity. Left panel shows infectivity data. Upper right panel is a western blot showing levels of HIV-1 particles released into the supernatant (anti-MA and anti-CA detection). Middle right panel shows cellular expression levels and proteolytic processing of Gag (anti-MA and anti-CA detection). Lower right panel shows cellular MVB12A expression levels (anti-Myc).
Comment in
- ESCRT-I part II: forming the real ESCRT-I complex.
Weissenhorn W, Göttlinger H. Weissenhorn W, et al. Cell Host Microbe. 2007 Jul 12;2(1):1-2. doi: 10.1016/j.chom.2007.06.006. Cell Host Microbe. 2007. PMID: 18005710
Similar articles
- The molecular basis for selective assembly of the UBAP1-containing endosome-specific ESCRT-I complex.
Wunderley L, Brownhill K, Stefani F, Tabernero L, Woodman P. Wunderley L, et al. J Cell Sci. 2014 Feb 1;127(Pt 3):663-72. doi: 10.1242/jcs.140673. Epub 2013 Nov 27. J Cell Sci. 2014. PMID: 24284069 Free PMC article. - Efficient cargo sorting by ESCRT-I and the subsequent release of ESCRT-I from multivesicular bodies requires the subunit Mvb12.
Curtiss M, Jones C, Babst M. Curtiss M, et al. Mol Biol Cell. 2007 Feb;18(2):636-45. doi: 10.1091/mbc.e06-07-0588. Epub 2006 Nov 29. Mol Biol Cell. 2007. PMID: 17135292 Free PMC article. - The human endosomal sorting complex required for transport (ESCRT-I) and its role in HIV-1 budding.
Stuchell MD, Garrus JE, Müller B, Stray KM, Ghaffarian S, McKinnon R, Kräusslich HG, Morham SG, Sundquist WI. Stuchell MD, et al. J Biol Chem. 2004 Aug 20;279(34):36059-71. doi: 10.1074/jbc.M405226200. Epub 2004 Jun 23. J Biol Chem. 2004. PMID: 15218037 - The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding - An overview.
Ahmed I, Akram Z, Iqbal HMN, Munn AL. Ahmed I, et al. Int J Biol Macromol. 2019 Apr 15;127:1-11. doi: 10.1016/j.ijbiomac.2019.01.015. Epub 2019 Jan 4. Int J Biol Macromol. 2019. PMID: 30615963 Review. - ESCRT & Co.
Roxrud I, Stenmark H, Malerød L. Roxrud I, et al. Biol Cell. 2010 Mar 12;102(5):293-318. doi: 10.1042/BC20090161. Biol Cell. 2010. PMID: 20222872 Review.
Cited by
- Membrane fission reactions of the mammalian ESCRT pathway.
McCullough J, Colf LA, Sundquist WI. McCullough J, et al. Annu Rev Biochem. 2013;82:663-92. doi: 10.1146/annurev-biochem-072909-101058. Epub 2013 Mar 18. Annu Rev Biochem. 2013. PMID: 23527693 Free PMC article. Review. - A helical assembly of human ESCRT-I scaffolds reverse-topology membrane scission.
Flower TG, Takahashi Y, Hudait A, Rose K, Tjahjono N, Pak AJ, Yokom AL, Liang X, Wang HG, Bouamr F, Voth GA, Hurley JH. Flower TG, et al. Nat Struct Mol Biol. 2020 Jun;27(6):570-580. doi: 10.1038/s41594-020-0426-4. Epub 2020 May 18. Nat Struct Mol Biol. 2020. PMID: 32424346 Free PMC article. - The role of cellular factors in promoting HIV budding.
Weiss ER, Göttlinger H. Weiss ER, et al. J Mol Biol. 2011 Jul 22;410(4):525-33. doi: 10.1016/j.jmb.2011.04.055. J Mol Biol. 2011. PMID: 21762798 Free PMC article. Review. - UBAP1: a new ESCRT member joins the cl_Ub.
Pashkova N, Piper RC. Pashkova N, et al. Structure. 2012 Mar 7;20(3):383-5. doi: 10.1016/j.str.2012.02.004. Structure. 2012. PMID: 22404994 Free PMC article. - Stepwise remodeling and subcompartment formation in individual vesicles by three ESCRT-III proteins.
Avalos-Padilla Y, Georgiev VN, Ewins E, Robinson T, Orozco E, Lipowsky R, Dimova R. Avalos-Padilla Y, et al. iScience. 2022 Dec 8;26(1):105765. doi: 10.1016/j.isci.2022.105765. eCollection 2023 Jan 20. iScience. 2022. PMID: 36590172 Free PMC article.
References
- Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases