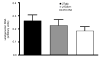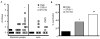CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I - PubMed (original) (raw)
CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I
Mark T Orr et al. Cell Host Microbe. 2007.
Abstract
In humans, herpes simplex virus (HSV) establishes latency in sensory nerve ganglia from where it periodically reactivates, whereas in murine models, the virus efficiently establishes latency but rarely reactivates. HSV inhibits MHC class I antigen presentation to CD8 T cells efficiently in humans but poorly in mice, and whether this is a crucial determinant of HSV's ability to reactivate in humans remains uncertain. To test this, we generated a panel of recombinant HSVs that inhibit presentation by murine MHC class I mimicking the effect in humans. Antigen-specific CD8 T cells prevent the in vivo reactivation of wild-type HSV. Despite their presence in the ganglia of latently infected mice, CD8 T cells do not prevent the reactivation of recombinant HSVs that inhibit murine MHC class I in mice. These findings suggest that efficient inhibition of MHC class I by HSV is a key factor in its ability to reactivate in humans.
Figures
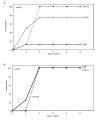
Figure 1. Viral inhibition of MHC class I does not prolong or prevent the clearance of primary infection
Female C57Bl/6 mice were infected with 2×105 pfu/eye of the indicated viruses. (A) 8 mice/virus were serially swabbed for viral shedding from both eyes on the indicated days. Alternatively, on the indicated days 4 mice/virus were euthanized and the (B) eyes and (C) trigeminal ganglia were harvested. Viral titers were determined by plaque formation on Vero cells. Statistically significant increases in viral titer compared to 27gfp on the same day are indicated (* p<0.05 and ** p<0.01). Error bars = one SEM. n.d. = not detected.
Figure 2. The amount of latent viral DNA is unaltered by viral inhibition of MHC class I
The relative quantity of viral DNA in the trigeminal ganglia of latently-infected mice 2 weeks post-infection was determined by realtime PCR for viral genomic DNA and normalized to the number of copies of the murine adipsin gene in each sample. 16-24 ganglia/virus were analyzed. Error bars = one SEM. Data are pooled from 2 experiments with similar results. There were no statistically significant differences among the groups.
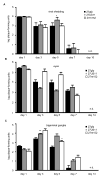
Figure 3. Viral inhibition of MHC class I reduces the number and activation state of HSV-specific CD8 T cells in latently-infected ganglia
HSV gB-specific CD8 T cells in latently-infected trigeminal ganglia at (A) 14-16 or (B and C) 32-46 days post-infection were identified (A and B) by binding of H-2Kb DimerX loaded with HSV gB498-505 peptide or (C) by gB peptide-induced IFN-γ production. (D) The activation status of HSV gB-specific CD8 T cells was determined as the percentage of H-2Kb-gB DimerX-positive CD8 T cells that contained intracellular granzyme B directly ex vivo. The cells shown in the plots are gated on total cells (A, B and C) or (D) CD8 T cells in the trigeminal ganglia. The bar graphs indicate the mean percentage ± SEM (A, B and C) of CD8 T cells that are specific for HSV gB or (D) of HSV gB-specific CD8 T cells that express granzyme B ex vivo. Data are pooled from (A) three, (B) five, (C) four and (D) two experiments with similar results; in each experiment 8-10 ganglia/virus were analyzed. The difference between mice infected with 27m152 compared to 27gfp in each panel was statistically significant (P<0.0001). Error bars = one SEM.
Figure 4. 27m152 and 27US11 reactivate in the presence of HSV-specific CD8 T cells
Reactivation of rHSVs in vitro from explanted, latently-infected trigeminal ganglia in the presence of effector HSV-specific CD8 T cells plus IL-2 and (A) IgG2b isotype control or (B) α-CD8 monoclonal antibody (n = 8 trigeminal ganglia/group). (A) The increased frequencies of reactivation for 27US11 (P = 0.01) and 27m152 (P = 0.0017) compared to 27gfp were statistically significant. (B) The results for 27gfp and 27US11 data were identical and thus the symbols for 27US11 are not apparent in the figure.
Figure 5. Viral inhibition of MHC class I promotes reactivation in vivo
In vivo reactivation from the trigeminal ganglia and eyes of latently-infected mice following UV-exposure 5 weeks post-infection. (A) The numbers of recovered pfu’s from the trigeminal ganglia and eyes are shown. In some mice virus was recovered from both sites, in others only in the trigeminal ganglia. Virus was never recovered from the eyes but not the trigeminal ganglia. Data represent 18-19 mice/virus and were pooled from 2 experiments with similar results. (B) The overall frequency of reactivation in the trigeminal ganglia for each virus and treatment group is shown. Data are from 27-31 mice/virus pooled from 3 experiments, including the two experiments shown in A and a third experiment in which reactivation was determined on a combined homogenate of the ganglia and eyes. Statistically significant increases versus 27gfp are indicated (* p<0.05 and ** p<0.01).
Figure 6. Inhibition of MHC class I abrogates CD8 T cell prevention of HSV reactivation
In vivo reactivation of rHSV following UV-exposure in 8-10 latently-infected mice treated with α-CD8 or IgG2b isotype control Ab. (A) The numbers of recovered pfu’s from both the trigeminal ganglia and eyes are shown. In some mice virus was recovered from both sites, in others only from the trigeminal ganglia. Virus was never recovered from the eyes but not the trigeminal ganglia. (B) The overall frequency of reactivation in the trigeminal ganglia for each virus and treatment group is indicated. The results shown are from one of two experiments performed with similar results in both.
Similar articles
- CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8+ TEM and CD8+ TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease.
Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, Bahraoui E, BenMohamed L. Srivastava R, et al. J Virol. 2017 Jun 26;91(14):e00278-17. doi: 10.1128/JVI.00278-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468883 Free PMC article. - Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia.
Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Khanna KM, et al. Immunity. 2003 May;18(5):593-603. doi: 10.1016/s1074-7613(03)00112-2. Immunity. 2003. PMID: 12753737 Free PMC article. - CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation.
St Leger AJ, Hendricks RL. St Leger AJ, et al. J Neurovirol. 2011 Dec;17(6):528-34. doi: 10.1007/s13365-011-0062-1. Epub 2011 Dec 8. J Neurovirol. 2011. PMID: 22161682 Review. - Control of HSV-1 latency in human trigeminal ganglia--current overview.
Held K, Derfuss T. Held K, et al. J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
- The Host-Pathogen Interplay: A Tale of Two Stories within the Cornea and Posterior Segment.
Dempsey MP, Conrady CD. Dempsey MP, et al. Microorganisms. 2023 Aug 12;11(8):2074. doi: 10.3390/microorganisms11082074. Microorganisms. 2023. PMID: 37630634 Free PMC article. Review. - Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease.
Morello CS, Levinson MS, Kraynyak KA, Spector DH. Morello CS, et al. J Virol. 2011 Apr;85(7):3461-72. doi: 10.1128/JVI.02521-10. Epub 2011 Jan 26. J Virol. 2011. PMID: 21270160 Free PMC article. - Development and pathogenic evaluation of recombinant herpes simplex virus type 1 expressing two fluorescent reporter genes from different lytic promoters.
Ramachandran S, Knickelbein JE, Ferko C, Hendricks RL, Kinchington PR. Ramachandran S, et al. Virology. 2008 Sep 1;378(2):254-64. doi: 10.1016/j.virol.2008.05.034. Epub 2008 Jul 11. Virology. 2008. PMID: 18619637 Free PMC article. - The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice.
Deng MM, Sun YW, Ding CM, Xu XY, Guo ZY, Han ZW, Lv CZ, Qi JK, Li YT, Yang X, Yu LY, Chen L. Deng MM, et al. Vaccines (Basel). 2022 Sep 23;10(10):1603. doi: 10.3390/vaccines10101603. Vaccines (Basel). 2022. PMID: 36298468 Free PMC article. - T cell response kinetics determines neuroinfection outcomes during murine HSV infection.
Lee AG, Scott JM, Fabbrizi MR, Jiang X, Sojka DK, Miller MJ, Baldridge MT, Yokoyama WM, Shin H. Lee AG, et al. JCI Insight. 2020 Mar 12;5(5):e134258. doi: 10.1172/jci.insight.134258. JCI Insight. 2020. PMID: 32161194 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD018184-25/HD/NICHD NIH HHS/United States
- T32 CA009537-19/CA/NCI NIH HHS/United States
- T32 CA009537-20/CA/NCI NIH HHS/United States
- HD18184/HD/NICHD NIH HHS/United States
- R01 HD018184-24/HD/NICHD NIH HHS/United States
- CA09537/CA/NCI NIH HHS/United States
- T32 CA009537/CA/NCI NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- R01 HD018184/HD/NICHD NIH HHS/United States
- R01 HD018184-26/HD/NICHD NIH HHS/United States
- R01 HD018184-27/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials