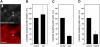Invasive and adherent bacterial pathogens co-Opt host clathrin for infection - PubMed (original) (raw)
Comparative Study
Invasive and adherent bacterial pathogens co-Opt host clathrin for infection
Esteban Veiga et al. Cell Host Microbe. 2007.
Abstract
Infection by the bacterium Listeria monocytogenes depends on host cell clathrin. To determine whether this requirement is widespread, we analyzed infection models using diverse bacteria. We demonstrated that bacteria that enter cells following binding to cellular receptors (termed "zippering" bacteria) invade in a clathrin-dependent manner. In contrast, bacteria that inject effector proteins into host cells in order to gain entry (termed "triggering" bacteria) invade in a clathrin-independent manner. Strikingly, enteropathogenic Escherichia coli (EPEC) required clathrin to form actin-rich pedestals in host cells beneath adhering bacteria, even though this pathogen remains extracellular. Furthermore, clathrin accumulation preceded the actin rearrangements necessary for Listeria entry. These data provide evidence for a clathrin-based entry pathway allowing internalization of large objects (bacteria and ligand-coated beads) and used by "zippering" bacteria as part of a general mechanism to invade host mammalian cells. We also revealed a nonendocytic role for clathrin required for extracellular EPEC infections.
Figures
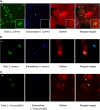
Figure 1. Localization of Endogenous Clathrin during Bacterial Infection
Extracellular bacteria, immunodetected before permeabilization, are shown in blue. Total (extracellular + intracellular) bacteria were detected after permeabilization and are shown in green. Endogenous clathrin was detected using X22 mAb (anticlathrin heavy chain) shown in red. (A) HeLa cells were infected for 10 min with E. coli (inv). Areas indicated by the arrows are magnified to better show clathrin surrounding the bacteria. (B) HeLa cells were infected for 20 min with S. aureus coated with fibronectin. (C) JEG3 cells were infected for 20 min with L. innocua (inlA). Scale bars, 2 μm.
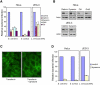
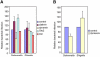
Figure 2. Role of Endocytic Machinery in Bacterial Entry
(A) HeLa and JEG3 cells KD by siRNA for the indicated proteins were infected with E. coli (inv), S. aureus, or L. innocua (inlA). Bacterial infection was measured by CFU (colony-forming unit) counts following gentamicin treatment. Cfu counts obtained from siRNA pretreated cells were normalized to control siRNA (RNA not targeting any cellular mRNA)-treated cells. (B) Protein KD by siRNA was tested by western-blot. In all cases, the left lane corresponds to the control cells and the right lane corresponds to the cells treated with siRNA against the indicated protein. Actin is shown as a loading control. (C) Cells were treated or not for 10 min with dynasore (80 μM) and then incubated with FITC-tagged transferrin (25 μg/ml) for 5 min, and transferrin was detected by fluorescence microscopy. (D) HeLa and JEG3 cells were treated or not (control) with 80 μM dynasore (added 10 min before infection) and infected with the indicated bacteria. Bacterial infection was measured by CFU count after gentamicin assay. CFUs observed from dynasore treated cells were normalized to control (untreated) cells. Error bars represent the standard deviation of at least 4 independent experiments.
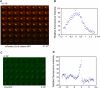
Figure 3. Clathrin Dynamics around Entering Bacteria/Beads
(A) Confocal time series (images acquired every ~1.6 s) from HeLa cells transiently expressing tdTomato-LCa (red) and infected with Listeria DH-L1039 expressing GFP (green). The figure shows every other acquisition frame from Movie S1. Scale bar, 2 μm. Bacterial fluorescence decreased during internalization. (B) Each dot represents the relative fluorescence intensity of tdTomato-LCa surrounding the bacterium at one time point from Movie S1. The maximal fluorescence intensity was arbitrarily considered as 100. (C) Confocal time series (images acquired every ~5.1 s) from HeLa cells transiently expressing EGFP-LCa (green). The figure shows one every other acquisition frame from Movie S7. Scale bar, 1 μm (D) Each dot represents the relative fluorescence intensity of EGFP-LCa surrounding the entering bead at a single time point from Movie S7. The maximal fluorescence intensity was arbitrarily considered as 100. Time is shown in minutes (’) and seconds (”). Time 0 corresponds to the maximal accumulation of clathrin at the site of entry of bacteria/beads.
Figure 4. Ligand-Induced Endocytosis of 1 μm Diameter Particles and Larger
Microspheres of 1 or 5.5 μm diameter coated with the indicated proteins were added to HeLa cells. EGF-coated beads (A) or fibronectin (FN)-coated beads (B) were added for 5 min to HeLa cells expressing tdTomato-LCa. Images were acquired at the contact zone of a bead and the cell; phase contrast images show the beads (left panels) and fluorescent images show clathrin (right pannels). Bars, 2 μm. (C, D, and E) Confocal orthogonal views (xy, xz, yz from a single optical section) of intracellular beads (green) and actin (red). The squares represent the xy view corresponding to the z plane indicated by the small arrowheads in the lateral rectangles. The upper and lateral rectangles represent the xz and yz views, respectively. The x and y planes shown correspond to those indicated by the small arrowhead presented in the square. In both, the apical part of the cell looks inward and the lower part, in contact with the slide, to the outside of the figure. (F) Fibronectin or EGF-coated beads were added to HeLa cells treated or not (control) with dynasore. The number of internalized beads is represented with respect to the total beads counted that was taken as 100. A minimum of 300 cells were examined for each condition.
Figure 5. Role of Clathrin in Actin Polymerization around Entering Bacteria
(A) InlB coated beads colocalize with clathrin in the presence of cytochalasin D. Cells were pretreated with cytochalasin D and incubated for 15 min with InlB-coated beads. The figure shows beads imaged by phase contrast (upper panel) and fluorescent images of clathrin (lower panel). Bar, 2 μm. (B) HeLa cells were treated or not (control) with cytochalasin D and incubated with Listeria for 5 min. The number of Listeria colocalizing with clathrin was then counted. Data are shown as relative values with respect to colocalization in control cells, considered as 100. The experiment was repeated four times and a minimum of 200 cells were analyzed in each individual experiment. (C and D) HeLa cells were treated with siRNA against dynamin, clathrin, or with control siRNA and incubated with Listeria for 5 min. Listeria colocalizing with actin was then counted. Data are shown as relative values with respect to control, considered as 100. The experiments were repeated four times and a minimum of 200 cells were analyzed in each experiment. Error bars represent the standard deviation.
Figure 6. Role of Endocytic Machinery in Entry of Triggering Bacteria
HeLa cells with the indicated proteins KD by siRNA (A) or pretreated with 80 μM dynasore (10 min before infection) (B) were infected with S. typhimurium or S. flexneri. Bacterial infection was measured by CFU count after gentamicin treatment. CFUs observed from siRNA or dynasore pretreated cells were normalized to controls, i.e., RNAi not targeting any mRNA (A) and untreated cells (B). These experiments were performed in parallel to those shown in Figure 2. Error bars represent the standard deviation of at least 4 independent experiments.
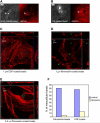
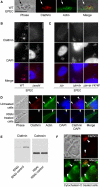
Figure 7. Clathrin Associated with EPEC
(A) Wild-type EPEC infected HeLa cells stained for clathrin and actin. Scale bar, 5 μm. Arrows points to a bacterium that is not attached to the-cell. Positive staining is not associated with the nonattached bacterium. (B) Clathrin immunolocalization on wild-type and Δ_escN_ EPEC infected cells. Clusters of bacteria are visible in the middle row of panels. Scale bar, 10 μm. (C) Clathrin immunolocalization on Δ_tir_ EPEC, Δ_tir_ EPEC complemented with EPEC tir (Δ_tir_ EPEC + EPEC tir), and Δ_tir_ EPEC complemented with tir that has a point mutation at tyrosine 474 (Δ_tir_ EPEC + tir Y474F). Clusters of bacteria are visible in the middle row of panels. Scale bar, 10 μm. (D) Untreated and clathrin RNAi knocked-down cells stained for clathrin, actin, and DAPI. Arrowheads indicate the location of some attached bacteria. Scale bar, 10 μm. (E) Western blot of clathrin knockdown and control RNAi treated HeLa cells. The blot was immunoreacted with the anti-clathrin antibody used in (A)–(D), then stripped and reprobed with an anti-calnexin antibody to compare the protein loading levels. (F) Cytochalasin D pretreated HeLa cells infected with EPEC and immunostained for clathrin and actin. Arrowheads indicate the location of some attached bacteria. Scale bar, 10 μm.
Comment in
- Clathrin: an amazing multifunctional dreamcoat?
Pauly BS, Drubin DG. Pauly BS, et al. Cell Host Microbe. 2007 Nov 15;2(5):288-90. doi: 10.1016/j.chom.2007.10.007. Cell Host Microbe. 2007. PMID: 18005749 Review.
Similar articles
- Non-classical use of clathrin during bacterial infections.
Cossart P, Veiga E. Cossart P, et al. J Microsc. 2008 Sep;231(3):524-8. doi: 10.1111/j.1365-2818.2008.02065.x. J Microsc. 2008. PMID: 18755008 Review. - Role for CD2AP and other endocytosis-associated proteins in enteropathogenic Escherichia coli pedestal formation.
Guttman JA, Lin AE, Veiga E, Cossart P, Finlay BB. Guttman JA, et al. Infect Immun. 2010 Aug;78(8):3316-22. doi: 10.1128/IAI.00161-10. Epub 2010 Jun 1. Infect Immun. 2010. PMID: 20515931 Free PMC article. - The role of clathrin-dependent endocytosis in bacterial internalization.
Veiga E, Cossart P. Veiga E, et al. Trends Cell Biol. 2006 Oct;16(10):499-504. doi: 10.1016/j.tcb.2006.08.005. Epub 2006 Sep 8. Trends Cell Biol. 2006. PMID: 16962776 Free PMC article. Review. - Cytoskeleton rearrangements during Listeria infection: clathrin and septins as new players in the game.
Mostowy S, Cossart P. Mostowy S, et al. Cell Motil Cytoskeleton. 2009 Oct;66(10):816-23. doi: 10.1002/cm.20353. Cell Motil Cytoskeleton. 2009. PMID: 19296488 Review. - Eps15 and Epsin1 are crucial for enteropathogenic Escherichia coli pedestal formation despite the absence of adaptor protein 2.
Lin AE, Benmerah A, Guttman JA. Lin AE, et al. J Infect Dis. 2011 Sep 1;204(5):695-703. doi: 10.1093/infdis/jir386. Epub 2011 Aug 2. J Infect Dis. 2011. PMID: 21810914
Cited by
- Molecular mechanisms regulating formation, trafficking and processing of annular gap junctions.
Falk MM, Bell CL, Kells Andrews RM, Murray SA. Falk MM, et al. BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):22. doi: 10.1186/s12860-016-0087-7. BMC Cell Biol. 2016. PMID: 27230503 Free PMC article. Review. - Shigella flexneri utilize the spectrin cytoskeleton during invasion and comet tail generation.
Ruetz TJ, Lin AE, Guttman JA. Ruetz TJ, et al. BMC Microbiol. 2012 Mar 16;12:36. doi: 10.1186/1471-2180-12-36. BMC Microbiol. 2012. PMID: 22424399 Free PMC article. - Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells.
Redelman-Sidi G, Iyer G, Solit DB, Glickman MS. Redelman-Sidi G, et al. Cancer Res. 2013 Feb 1;73(3):1156-67. doi: 10.1158/0008-5472.CAN-12-1882. Cancer Res. 2013. PMID: 23378476 Free PMC article. - Listeria monocytogenes Co-Opts the Host Exocyst Complex To Promote Internalin A-Mediated Entry.
Gyanwali GC, Herath TUB, Gianfelice A, Ireton K. Gyanwali GC, et al. Infect Immun. 2022 Dec 15;90(12):e0032622. doi: 10.1128/iai.00326-22. Epub 2022 Oct 18. Infect Immun. 2022. PMID: 36255255 Free PMC article. - Tannerella forsythia invasion in oral epithelial cells requires phosphoinositide 3-kinase activation and clathrin-mediated endocytosis.
Mishima E, Sharma A. Mishima E, et al. Microbiology (Reading). 2011 Aug;157(Pt 8):2382-2391. doi: 10.1099/mic.0.048975-0. Epub 2011 May 26. Microbiology (Reading). 2011. PMID: 21622527 Free PMC article.
References
- Agerer F, Lux S, Michel A, Rohde M, Ohlsen K, Hauck CR. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 2005;118:2189–2200. - PubMed
- Braun L, Ohayon H, Cossart P. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 1998;27:1077–1087. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources