Cysteine 111 affects aggregation and cytotoxicity of mutant Cu,Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis - PubMed (original) (raw)
Cysteine 111 affects aggregation and cytotoxicity of mutant Cu,Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis
Mauro Cozzolino et al. J Biol Chem. 2008.
Abstract
Converging evidence indicates that aberrant aggregation of mutant Cu,Zn-superoxide dismutase (mutSOD1) is strongly implicated in familial amyotrophic lateral sclerosis (FALS). MutSOD1 forms high molecular weight oligomers, which disappear under reducing conditions, both in neural tissues of FALS transgenic mice and in transfected cultured cells, indicating a role for aberrant intermolecular disulfide cross-linking in the oligomerization and aggregation process. To study the contribution of specific cysteines in the mechanism of aggregation, we mutated human SOD1 in each of its four cysteine residues and, using a cell transfection assay, analyzed the solubility and aggregation of those SOD1s. Our results suggest that the formation of mutSOD1 aggregates are the consequence of covalent disulfide cross-linking and non-covalent interactions. In particular, we found that the removal of Cys-111 strongly reduces the ability of a range of different FALS-associated mutSOD1s to form aggregates and impair cell viability in cultured NSC-34 cells. Moreover, the removal of Cys-111 impairs the ability of mutSOD1s to form disulfide cross-linking. Treatments that deplete the cellular pool of GSH exacerbate mutSOD1s insolubility, whereas an overload of intracellular GSH or overexpression of glutaredoxin-1, which specifically catalyzes the reduction of protein-SSG-mixed disulfides, significantly rescues mutSOD1s solubility. These data are consistent with the view that the redox environment influences the oligomerization/aggregation pathway of mutSOD1 and point to Cys-111 as a key mediator of this process.
Figures
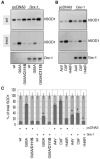
FIGURE 1. The effect of cysteine mutations on the solubility of human SOD1
A, NSC-34/pTetON cells were transfected with pTRE plasmids coding for wtSOD1 or the indicated mutSOD1s. To induce the expression of SOD1, 1 _μ_g/ml doxycycline was added. After 48 h of culture, insoluble (ins) and soluble (sol) fractions were isolated as described under “Experimental Procedures.” Equal volumes from each fraction were subjected to standard, reducing SDS-PAGE and analyzed by Western blotting with an antibody anti-SOD1. Transient transfection elicits high expression levels of human SOD1, and endogenous mouse SOD1 is detectable only after longer exposure. nt, untransfected cells. One typical experiment is shown. B, histogram of the distribution of SOD1s between the soluble and insoluble fractions in NSC-34 cells as determined in n = 4 independent Western blot experiments. The total expression level was considered for each line as 100%, and the soluble and insoluble percentages from data in densitometric arbitrary units are expressed as mean ± S.D.
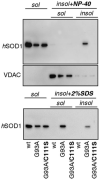
FIGURE 2. MutSOD1 insolubility is reversed by extraction with SDS, but not with non-ionic detergents
NSC-34/pTetON cells were transfected with pTRE plasmids coding for the indicated SOD1s in the presence of 1 _μ_g/ml doxycycline. Cells were mechanically lysed in a buffer without any detergents, and the soluble and insoluble fractions were isolated. Insoluble fractions were then resuspended in a buffer containing 0.5% Nonidet P-40 (upper panel) or 2% SDS (lower panel). In this last case, lysates were heated to 100 °C for 5 min. Detergent-insoluble proteins were resuspended in Laemmli sample buffer. Equivalent amounts of each fraction were subjected to standard, reducing SDS-PAGE and analyzed by Western blotting with an anti-SOD1 antibody or an antibody recognizing the transmembrane mitochondrial protein voltage-dependent anion channel.
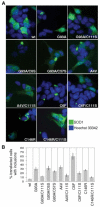
FIGURE 3. C111S mutation inhibits the formation of intracellular aggregates by mutSOD1s
A, NSC-34 cells were transfected with plasmids coding for wtSOD1 and the indicated mutSOD1s. After 48 h of culture, cells were subjected to immunofluorescence analysis with an antibody anti-SOD1. B, the proportion of SOD1-transfected cells bearing intracytoplasmic inclusions was scored (n = 3).
FIGURE 4. The replacement of Cys-111 protects NSC-34 cells from mutSOD1-induced cell toxicity
NSC-34/pTetON cells were left untransfected (nt) or transfected with pTRE plasmids coding for wtSOD1 and the indicated mutSOD1s in the absence (ctrl) or presence of 1 _μ_g/ml doxycycline. After 48 h of culture, cell viability was assessed by an MTS assay. Absorbances at 490 nm are expressed as the percentage of the relative control cells. Values significantly different from relative controls are indicated with an asterisk when p < 0.01 (n = 3).
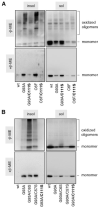
FIGURE 5. G93A and C6F mutSOD1s, but not G93A/C111S or C6F/C111S mutSOD1, accumulate as a disulfide-linked oligomeric form in the insoluble fractions of NSC-34 transfected cells
A, soluble and insoluble fractions from NSC-34 cells transiently transfected as indicated, were collected as described. Insoluble fractions were solubilized in a buffer containing 2% SDS. Soluble and insoluble fractions were then boiled for 5 min at 100 °C in the presence of 100 m
m
iodoacetamide. 15 _μ_g of proteins from each sample was subjected to a denaturing PAGE, either non-reducing (without _β_-mercaptoethanol, β-ME, upper panel) or reducing (with β-ME, lower panel). Western blot analysis was performed with an anti-SOD1 antibody. B, soluble and insoluble fractions from NSC-34 cell transiently transfected as indicated were treated as in A and analyzed by Western blotting with an anti-SOD1 antibody.
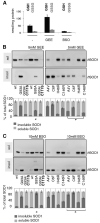
FIGURE 6. Redox control of mutant SOD1 aggregation in NSC-34 cells
A, reduced (GSH) and oxidized (GSSG) glutathione levels were measured by high performance liquid chromatography after a 48-h treatment of NSC-34 cells with 5 m
m
GEE or 10 m
m
BSO. B, NSC-34/pTetON cells were transiently transfected as indicated in the absence or in the presence of 5 m
m
glutathione ethyl ester (GEE). After 48 h, soluble and insoluble fractions were isolated and analyzed by Western blotting with an anti-SOD1 antibody. C, cells were transfected as in B and left untreated or treated with 10 m
m l
-buthionine-(S,R)-sulfoximine (BSO) for 48 h. Soluble and insoluble fractions were isolated and analyzed by Western blotting with an anti-SOD1 antibody. Histograms are calculated as in Fig. 1. Asterisks indicate values of insoluble mutSOD1s significantly different (p < 0.01, n = 3) from relative, untreated controls.
FIGURE 7. The overexpression of glutaredoxin-1 diminishes mutSOD1s insolubility
A and B, NSC-34/pTetON cells were transfected with wild-type or mutant SOD1s in the presence of a control plasmid (pcDNA3) or a plasmid coding for mouse cytosolic glutaredoxin-1 (Grx-1). Plasmids for SOD1 and Grx-1 were in a ratio of 1:3. Soluble and insoluble fractions were isolated after 48 h of culture in the presence of 1 _μ_g/ml doxycycline, and equivalent amounts were analyzed by Western blotting with an antibody anti-SOD1. An anti-Grx-1 antibody was used to reveal the levels of expression of Grx-1 in the soluble fraction. C, histograms are calculated as in Fig. 1. Asterisks indicate values of insoluble mutSOD1s significantly different (p < 0.01, n = 3) from relative controls.
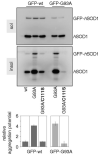
FIGURE 8. Cys-111 mediates the transfer of the aggregated phenotype from G93A mutSOD1 to wtSOD1
wtSOD1 and the mutSOD1 G93A and G93A/C111S were co-transfected for 48 h with GFP-tagged versions of wtSOD1 and the G93A mutant in NSC-34/pTetON cells. Soluble and insoluble fractions were isolated and analyzed by Western blotting with an anti-SOD1 antibody. Positions of GFP-tagged and untagged human SOD1s are indicated. Filters were stripped and reprobed with an anti-GFP antibody to check for the specificity of GFP-SOD1 signals (not shown). Histogram of the distribution of GFP-SOD1s in the insoluble fractions of NSC-34 cells as determined in n = 3 independent Western blot experiments. The relative levels of insoluble GFP-SOD1s were expressed as mean ± S.D., considering GFP-wtSOD1 as one unit.
Similar articles
- Oligomerization of mutant SOD1 in mitochondria of motoneuronal cells drives mitochondrial damage and cell toxicity.
Cozzolino M, Pesaresi MG, Amori I, Crosio C, Ferri A, Nencini M, Carrì MT. Cozzolino M, et al. Antioxid Redox Signal. 2009 Jul;11(7):1547-58. doi: 10.1089/ars.2009.2545. Antioxid Redox Signal. 2009. PMID: 19344252 - Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction.
Tiwari A, Hayward LJ. Tiwari A, et al. J Biol Chem. 2003 Feb 21;278(8):5984-92. doi: 10.1074/jbc.M210419200. Epub 2002 Nov 27. J Biol Chem. 2003. PMID: 12458194 - Mutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic mice.
Tobisawa S, Hozumi Y, Arawaka S, Koyama S, Wada M, Nagai M, Aoki M, Itoyama Y, Goto K, Kato T. Tobisawa S, et al. Biochem Biophys Res Commun. 2003 Apr 4;303(2):496-503. doi: 10.1016/s0006-291x(03)00353-x. Biochem Biophys Res Commun. 2003. PMID: 12659845 - Structure, folding, and misfolding of Cu,Zn superoxide dismutase in amyotrophic lateral sclerosis.
Rakhit R, Chakrabartty A. Rakhit R, et al. Biochim Biophys Acta. 2006 Nov-Dec;1762(11-12):1025-37. doi: 10.1016/j.bbadis.2006.05.004. Epub 2006 May 22. Biochim Biophys Acta. 2006. PMID: 16814528 Review. - Motoneuronal and muscle-selective removal of ALS-related misfolded proteins.
Crippa V, Galbiati M, Boncoraglio A, Rusmini P, Onesto E, Giorgetti E, Cristofani R, Zito A, Poletti A. Crippa V, et al. Biochem Soc Trans. 2013 Dec;41(6):1598-604. doi: 10.1042/BST20130118. Biochem Soc Trans. 2013. PMID: 24256261 Review.
Cited by
- Redox modification of cell signaling in the cardiovascular system.
Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Shao D, et al. J Mol Cell Cardiol. 2012 Mar;52(3):550-8. doi: 10.1016/j.yjmcc.2011.09.009. Epub 2011 Sep 17. J Mol Cell Cardiol. 2012. PMID: 21945521 Free PMC article. Review. - Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria.
Kawamata H, Manfredi G. Kawamata H, et al. Hum Mol Genet. 2008 Nov 1;17(21):3303-17. doi: 10.1093/hmg/ddn226. Epub 2008 Aug 13. Hum Mol Genet. 2008. PMID: 18703498 Free PMC article. - Cytotoxicity of superoxide dismutase 1 in cultured cells is linked to Zn2+ chelation.
Johansson AS, Vestling M, Zetterström P, Lang L, Leinartaitė L, Karlström M, Danielsson J, Marklund SL, Oliveberg M. Johansson AS, et al. PLoS One. 2012;7(4):e36104. doi: 10.1371/journal.pone.0036104. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558346 Free PMC article. - A novel variant of human superoxide dismutase 1 harboring amyotrophic lateral sclerosis-associated and experimental mutations in metal-binding residues and free cysteines lacks toxicity in vivo.
Prudencio M, Lelie H, Brown HH, Whitelegge JP, Valentine JS, Borchelt DR. Prudencio M, et al. J Neurochem. 2012 May;121(3):475-85. doi: 10.1111/j.1471-4159.2012.07690.x. Epub 2012 Mar 20. J Neurochem. 2012. PMID: 22332887 Free PMC article. - Computational approaches to understanding protein aggregation in neurodegeneration.
Redler RL, Shirvanyants D, Dagliyan O, Ding F, Kim DN, Kota P, Proctor EA, Ramachandran S, Tandon A, Dokholyan NV. Redler RL, et al. J Mol Cell Biol. 2014 Apr;6(2):104-15. doi: 10.1093/jmcb/mju007. Epub 2014 Mar 11. J Mol Cell Biol. 2014. PMID: 24620031 Free PMC article. Review.
References
- Carri MT, Grignaschi G, Bendotti C. Trends Pharmacol. Sci. 2006;27:267–273. - PubMed
- Shaw BF, Valentine JS. Trends Biochem. Sci. 2007;32:78–85. - PubMed
- Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Hum. Mol. Genet. 2003;12:2753–2764. - PubMed
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Science. 1998;281:1851–1854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous