Orai1 subunit stoichiometry of the mammalian CRAC channel pore - PubMed (original) (raw)
Orai1 subunit stoichiometry of the mammalian CRAC channel pore
Olivier Mignen et al. J Physiol. 2008.
Abstract
Agonist-activated Ca2+ entry plays a critical role in Ca2+ signalling in non-excitable cells. One mode of such entry is activated as a consequence of the depletion of intracellular Ca2+ stores. This depletion is sensed by the protein STIM1 in the endoplasmic reticulum, which then translocates to regions close to the plasma membrane where it induces the activation of store-operated conductances. The most thoroughly studied of these conductances are the Ca2+ release-activated Ca2+ (CRAC) channels, and recent studies have identified the protein Orai1 as comprising the essential pore-forming subunit of these channels. Although evidence suggests that Orai1 can assemble as homomultimers, whether this assembly is necessary for the formation of functional CRAC channels and, if so, their relevant stoichiometry is unknown. To examine this, we have used an approach involving the expression of preassembled tandem Orai1 multimers comprising different numbers of subunits into cells stably overexpressing STIM1, followed by the recording of maximally activated CRAC channel currents. In each case, any necessity for recruitment of additional Orai1 units to these preassembled multimers in order to form functional channels was evaluated by coexpression with a dominant-negative Orai1 mutant. In this way we were able to demonstrate, for the first time, that the functional CRAC channel pore is formed by a tetrameric assembly of Orai1 subunits.
Figures
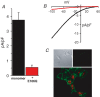
Figure 1. The E106Q mutant Orai1 inhibits CRAC channel currents in STIM1- stable cells stably expressing wild-type Orai1
A, inward CRAC channel currents measured at −40 mV in STIM1-stable cells stably expressing the wild-type Orai1 either alone (black bar) or after transfection with the E106Q mutant Orai1 (red bar). Data are presented as means ±
s.e.m.
; n = 15 and 10, respectively. B, representative current–voltage relationships for CRAC channel currents measured in STIM1-stable cells stably expressing wild-type Orai1 monomers either alone (black trace), or after transfection of the E106Q mutant Orai1 (red trace). C, upper panels, images showing the absence of significant background fluorescence in untransfected STIM1-stable cells. Shown is a DIC image of STIM1-stable cells (left panel), and the same field in confocal mode following immunocytochemistry with the anti-FLAG antibody (right panel). Lower panel, confocal image showing that transfection with the E106Q mutant Orai1 in STIM1-stable cells stably expressing the Orai1 monomer does not affect the plasma membrane localization of Orai1. Orai1 is detected in the confocal image by an antibody targeting the C-terminal FLAG tag and shown in green. Those cells transfected with the E106Q mutant are distinguished by cotransfection of a nuclear-targeted HcRed plasmid (pHcRed1-N1, Clontech, Palo Alto, CA, USA).
Figure 2. Predicted composition of expressed channel stoichiometries in cells coexpressing the E106Q mutant Orai1 and each of the tandem Orai1 multimers
Wild-type Orai1 subunits (blue circles) and E106Q Orai1 (black circles) are shown for each transfected multimer (dimer, trimer or tetramer), together with the predicted possible combinations of the transfected subunits based on the stoichiometry of functional CRAC channels being either a dimer, a trimer, or a tetramer. Those assemblies predicted to result in functional channels are shown with a central ‘pore’ in red, and predicted non-functional channels are represented with a pore in white. The consequent predicted overall effect on CRAC channel currents in the presence of the E106Q mutant is indicated. See text for details.
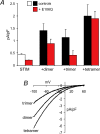
Figure 3. Effect of expression of tandem Orai1 multimers with and without the E106Q mutant Orai1
A, inward CRAC channel currents measured at −40 mV in untransfected STIM1-stable cells (white bar) and after transfection of the wild-type Orai1 multimers either alone (black bars) or along with 0.75 μg DNA of the E106Q mutant Orai1 (red bars). Data are presented as means ±
s.e.m.
; n = 9–13. B, representative current–voltage relationships for CRAC channel currents measured in STIM1-stable cells after transfection of the respective wild-type Orai1 multimers.
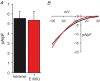
Figure 4. Expression of the E106Q mutant has no effect on CRAC channel currents in cells stably expressing the Orai1 tetramer
A, inward CRAC channel currents measured at −40 mV in STIM1- stable cells stably expressing the wild-type Orai1 tetramer either alone (black bar) or after transfection with the E106Q mutant Orai1 (red bar). Data are presented as means ±
s.e.m.
; n = 12 and 7, respectively. B, representative current–voltage relationships for CRAC channel currents measured in STIM1-stable cells stably expressing wild-type Orai1 tetramer either alone (black trace), or after transfection of the E106Q mutant Orai1 (red trace).
Comment in
- Subunit stoichiometry and channel pore structure of ion channels: all for one, or one for one?
Cai X. Cai X. J Physiol. 2008 Feb 15;586(4):925-6. doi: 10.1113/jphysiol.2007.149153. Epub 2007 Dec 13. J Physiol. 2008. PMID: 18079155 Free PMC article. No abstract available.
Similar articles
- Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits.
Li Z, Liu L, Deng Y, Ji W, Du W, Xu P, Chen L, Xu T. Li Z, et al. Cell Res. 2011 Feb;21(2):305-15. doi: 10.1038/cr.2010.131. Epub 2010 Sep 14. Cell Res. 2011. PMID: 20838418 Free PMC article. - Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels.
Mignen O, Thompson JL, Shuttleworth TJ. Mignen O, et al. J Physiol. 2008 Jan 1;586(1):185-95. doi: 10.1113/jphysiol.2007.146258. Epub 2007 Nov 8. J Physiol. 2008. PMID: 17991693 Free PMC article. - Orai1 is an essential pore subunit of the CRAC channel.
Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Prakriya M, et al. Nature. 2006 Sep 14;443(7108):230-3. doi: 10.1038/nature05122. Epub 2006 Aug 20. Nature. 2006. PMID: 16921383 - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review. - On the stoichiometry of resting and activated CRAC channels.
Chen L, Xu T. Chen L, et al. Curr Top Membr. 2013;71:95-108. doi: 10.1016/B978-0-12-407870-3.00004-4. Curr Top Membr. 2013. PMID: 23890112 Review.
Cited by
- Ca(2+) release-activated Ca(2+) (CRAC) current, structure, and function.
Muik M, Schindl R, Fahrner M, Romanin C. Muik M, et al. Cell Mol Life Sci. 2012 Dec;69(24):4163-76. doi: 10.1007/s00018-012-1072-8. Epub 2012 Jul 17. Cell Mol Life Sci. 2012. PMID: 22802126 Free PMC article. Review. - Store-Operated Calcium Entry and Its Implications in Cancer Stem Cells.
Jardin I, Lopez JJ, Sanchez-Collado J, Gomez LJ, Salido GM, Rosado JA. Jardin I, et al. Cells. 2022 Apr 13;11(8):1332. doi: 10.3390/cells11081332. Cells. 2022. PMID: 35456011 Free PMC article. Review. - Subunit stoichiometry and channel pore structure of ion channels: all for one, or one for one?
Cai X. Cai X. J Physiol. 2008 Feb 15;586(4):925-6. doi: 10.1113/jphysiol.2007.149153. Epub 2007 Dec 13. J Physiol. 2008. PMID: 18079155 Free PMC article. No abstract available. - Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins.
Scrimgeour N, Litjens T, Ma L, Barritt GJ, Rychkov GY. Scrimgeour N, et al. J Physiol. 2009 Jun 15;587(Pt 12):2903-18. doi: 10.1113/jphysiol.2009.170662. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403622 Free PMC article. - Orai, STIM1 and iPLA2beta: a view from a different perspective.
Bolotina VM. Bolotina VM. J Physiol. 2008 Jul 1;586(13):3035-42. doi: 10.1113/jphysiol.2008.154997. Epub 2008 May 22. J Physiol. 2008. PMID: 18499724 Free PMC article.
References
- Cai X. Molecular evolution and structural analysis of the Ca2+ release-activated Ca2+ channel subunit, Orai. J Mol Biol. 2007;368:1284–1291. - PubMed
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. - PubMed
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-Hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. - PubMed
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous