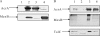Fitting periplasmic membrane fusion proteins to inner membrane transporters: mutations that enable Escherichia coli AcrA to function with Pseudomonas aeruginosa MexB - PubMed (original) (raw)
Fitting periplasmic membrane fusion proteins to inner membrane transporters: mutations that enable Escherichia coli AcrA to function with Pseudomonas aeruginosa MexB
Ganesh Krishnamoorthy et al. J Bacteriol. 2008 Jan.
Abstract
AcrAB-TolC from Escherichia coli is a multidrug efflux complex capable of transenvelope transport. In this complex, AcrA is a periplasmic membrane fusion protein that establishes a functional connection between the inner membrane transporter AcrB of the RND superfamily and the outer membrane channel TolC. To gain insight into the mechanism of the functional association between components of this complex, we replaced AcrB with its close homolog MexB from Pseudomonas aeruginosa. Surprisingly, we found that AcrA is promiscuous and can form a partially functional complex with MexB and TolC. The chimeric AcrA-MexB-TolC complex protected cells from sodium dodecyl sulfate, novobiocin, and ethidium bromide but failed with other known substrates of MexB. We next identified single and double mutations in AcrA and MexB that enabled the complete functional fit between AcrA, MexB, and TolC. Mutations in either the alpha-helical hairpin of AcrA making contact with TolC or the beta-barrel domain lying on MexB improved the functional alignment between components of the complex. Our results suggest that three components of multidrug efflux pumps do not associate in an "all-or-nothing" fashion but accommodate a certain degree of flexibility. This flexibility in the association between components affects the transport efficiency of RND pumps.
Figures
FIG. 1.
AcrA, MexB, and TolC form a tripartite multidrug efflux complex. (A) Expression of AcrA and MexB in E. coli W4680AD cells. Membrane fractions were isolated from W4680AD cells (lane 1), W4680AD cells producing chimeric AcrA-MexB (lane 2), wild-type AcrA-AcrB cells (lane 3), and wild-type MexAB-OprM cells (lane 4). Equal amounts of the total membrane protein were separated on 10% SDS-PAGE and analyzed by immunoblotting with anti-AcrA (top panel) and anti-MexB (bottom panel) antibodies. Arrows indicate AcrA and MexB proteins. (B) MexB and TolC were copurified with AcrA. AcrA was purified using metal-affinity chromatography from membrane fractions of cells producing AcrA-AcrB (lane 1), MexAB-OprM (lane 2), AcrA-MexB (lane 3), or AcrA only (lane 4). Samples were analyzed by SDS-PAGE, followed by immunoblotting with anti-AcrA (top panel), anti-MexB (middle panel), and anti-TolC antibodies (bottom panel).
FIG. 2.
Isolated AcrA-MexB mutants are expressed at the same level in E. coli W4680AD cells. Equal amounts of total membrane protein isolated from cells carrying mutant AcrA-MexB and wild-type AcrA-MexB variants were separated on 10% SDS-PAGE and analyzed by immunoblotting with anti-AcrA (top panel) and anti-MexB (bottom panel) antibodies. Arrows indicate AcrA and MexB proteins.
FIG. 3.
Positions of amino acid substitutions in AcrA and MexB that enhance the activity of chimeric AcrA-MexB. (A) Three-dimensional structure of monomeric AcrA (24), with mutated residues shown as red spheres. (B) The homology model of MexB based on the AcrB crystal structure (26) was generated using Swiss-Model software (
http://swissmodel.expasy.org//SWISS-MODEL.html
), with the mutated residues shown as spheres.
FIG. 4.
MICs do not correlate with the amounts of MexB and TolC copurified with AcrAHis. AcrAHis was purified from cells producing the wild-type and the AcrA-MexB-TolC mutant complexes. The amounts of copurified proteins were analyzed by immunoblotting with anti-AcrA (top panel), anti-MexB (middle panel), and anti-TolC (bottom panel) antibodies. Lanes 1, AcrA-MexB; 2, AcrA(D111N)-MexB; 3, AcrA(G240S)-MexB; 4, AcrA(V244M)-MexB; 5, AcrA(S249N)-MexB; 6, AcrA-MexB(T489I); 7, AcrA-MexB(T557I); 8, AcrA-MexB(A802V); 9, AcrA(V244M)-MexB(T489I); 10, AcrA(V244M)-MexB(T557I); 11, AcrA-MexB(T329I); 12, AcrA-MexB(T329I/A802V).
Similar articles
- AcrB-AcrA Fusion Proteins That Act as Multidrug Efflux Transporters.
Hayashi K, Nakashima R, Sakurai K, Kitagawa K, Yamasaki S, Nishino K, Yamaguchi A. Hayashi K, et al. J Bacteriol. 2015 Nov 2;198(2):332-42. doi: 10.1128/JB.00587-15. Print 2016 Jan 15. J Bacteriol. 2015. PMID: 26527645 Free PMC article. - The C-terminal domain of AcrA is essential for the assembly and function of the multidrug efflux pump AcrAB-TolC.
Ge Q, Yamada Y, Zgurskaya H. Ge Q, et al. J Bacteriol. 2009 Jul;191(13):4365-71. doi: 10.1128/JB.00204-09. Epub 2009 May 1. J Bacteriol. 2009. PMID: 19411330 Free PMC article. - Genetic evidence for functional interactions between TolC and AcrA proteins of a major antibiotic efflux pump of Escherichia coli.
Gerken H, Misra R. Gerken H, et al. Mol Microbiol. 2004 Nov;54(3):620-31. doi: 10.1111/j.1365-2958.2004.04301.x. Mol Microbiol. 2004. PMID: 15491355 - The AcrB efflux pump: conformational cycling and peristalsis lead to multidrug resistance.
Seeger MA, Diederichs K, Eicher T, Brandstätter L, Schiefner A, Verrey F, Pos KM. Seeger MA, et al. Curr Drug Targets. 2008 Sep;9(9):729-49. doi: 10.2174/138945008785747789. Curr Drug Targets. 2008. PMID: 18781920 Review. - AcrAB and related multidrug efflux pumps of Escherichia coli.
Nikaido H, Zgurskaya HI. Nikaido H, et al. J Mol Microbiol Biotechnol. 2001 Apr;3(2):215-8. J Mol Microbiol Biotechnol. 2001. PMID: 11321576 Review.
Cited by
- The Art of War with Pseudomonas aeruginosa: Targeting Mex Efflux Pumps Directly to Strategically Enhance Antipseudomonal Drug Efficacy.
Avakh A, Grant GD, Cheesman MJ, Kalkundri T, Hall S. Avakh A, et al. Antibiotics (Basel). 2023 Aug 9;12(8):1304. doi: 10.3390/antibiotics12081304. Antibiotics (Basel). 2023. PMID: 37627724 Free PMC article. Review. - Common recognition topology of mex transporters of Pseudomonas aeruginosa revealed by molecular modelling.
Catte A, K Ramaswamy V, Vargiu AV, Malloci G, Bosin A, Ruggerone P. Catte A, et al. Front Pharmacol. 2022 Nov 11;13:1021916. doi: 10.3389/fphar.2022.1021916. eCollection 2022. Front Pharmacol. 2022. PMID: 36438787 Free PMC article. - Molecular Determinants for OMF Selectivity in Tripartite RND Multidrug Efflux Systems.
Boyer E, Dessolin J, Lustig M, Decossas M, Phan G, Cece Q, Durand G, Dubois V, Sansen J, Taveau JC, Broutin I, Daury L, Lambert O. Boyer E, et al. Antibiotics (Basel). 2022 Jan 18;11(2):126. doi: 10.3390/antibiotics11020126. Antibiotics (Basel). 2022. PMID: 35203729 Free PMC article. - A Model for Allosteric Communication in Drug Transport by the AcrAB-TolC Tripartite Efflux Pump.
Webber A, Ratnaweera M, Harris A, Luisi BF, Ntsogo Enguéné VY. Webber A, et al. Antibiotics (Basel). 2022 Jan 1;11(1):52. doi: 10.3390/antibiotics11010052. Antibiotics (Basel). 2022. PMID: 35052929 Free PMC article. - Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria.
Alav I, Kobylka J, Kuth MS, Pos KM, Picard M, Blair JMA, Bavro VN. Alav I, et al. Chem Rev. 2021 May 12;121(9):5479-5596. doi: 10.1021/acs.chemrev.1c00055. Epub 2021 Apr 28. Chem Rev. 2021. PMID: 33909410 Free PMC article. Review.
References
- Akama, H., M. Kanemaki, M. Yoshimura, T. Tsukihara, T. Kashiwagi, H. Yoneyama, S. Narita, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J. Biol. Chem. 27952816-52819. - PubMed
- Akama, H., T. Matsuura, S. Kashiwagi, H. Yoneyama, S. Narita, T. Tsukihara, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 27925939-25942. - PubMed
- Andersen, C., C. Hughes, and V. Koronakis. 2001. Protein export and drug efflux through bacterial channel-tunnels. Curr. Opin. Cell Biol. 13412-416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases