Nucleolin regulates c-Jun/Sp1-dependent transcriptional activation of cPLA2alpha in phorbol ester-treated non-small cell lung cancer A549 cells - PubMed (original) (raw)
Nucleolin regulates c-Jun/Sp1-dependent transcriptional activation of cPLA2alpha in phorbol ester-treated non-small cell lung cancer A549 cells
Jen-Hui Tsou et al. Nucleic Acids Res. 2008 Jan.
Abstract
The expression of cPLA2 is critical for transformed growth of non-small cell lung cancer (NSCLC). It is known that phorbol 12-myristate 13-acetate (PMA)-activated signal transduction pathway is thought to be involved in the oncogene action in NSCLC and enzymatic activation of cPLA2. However, the transcriptional regulation of cPLA2alpha in PMA-activated NSCLC is not clear. In this study, we found that PMA induced the mRNA level and protein expression of cPLA2alpha. In addition, two Sp1-binding sites of cPLA2alpha promoter were required for response to PMA and c-Jun overexpression. Small interfering RNA (siRNA) of c-Jun and nucleolin inhibited PMA induced the promoter activity and protein expression of cPLA2alpha. Furthermore, PMA stimulated the formation of c-Jun/Sp1 and c-Jun/nucleolin complexes as well as the binding of these transcription factor complexes to the cPLA2alpha promoter. Although Sp1-binding sites were required for the bindings of Sp1 and nucleolin to the promoter, the binding of nucleolin or Sp1 to the promoter was independent of each other. Our results revealed that c-Jun/nucleolin and c-Jun/Sp1 complexes play an important role in PMA-regulated cPLA2alpha gene expression. It is likely that nucleolin binding at place of Sp1 on gene promoter could also mediate the regulation of c-Jun/Sp1-activated genes.
Figures
Figure 1.
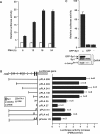
PMA induces the expression of cPLA2α in A549 cells. (A) A549 cells were starved for 18 h in serum-free culture medium and then treated with 5 nM PMA for a different time period as indicated. Northern blot analysis of mRNA was performed. (B) Cell lysates were subjected to SDS–PAGE and analyzed by western blotting with antibodies against cPLA2α and β-actin.
Figure 2.
Analysis of PMA-responsive element in the promoter region of _cPLA2_α gene in A549 cells. (A) Cells were transfected with 0.5 μg of luciferase plasmid bearing _cPLA2_α gene promoter by lipofection, incubated for 24 h and then treated with 5 nM PMA for a different time period as indicated. The luciferase activities and protein concentrations were determined and normalized. The results shown represent the means ± SEM of three determinations. Blank columns: non-treatment; black columns: PMA-treated cells. (B) Truncated promoter fragment was ligated into a luciferase plasmid as described in the Materials and Methods section. Numbers indicate the distance in base pairs from the translation start site. Cells were transfected with 0.5 μg of luciferase plasmid bearing _cPLA2_α gene promoter by lipofection, incubated for 24 h and then treated with 5 nM PMA for 18 h. The luciferase activities and protein concentrations were determined and normalized. The expression ratio of PMA treated to control cells is indicated. The results shown represent the means ± SEM of 3 to 8 independent experiments in triplicate wells for each construct. (C) Cells were transfected with 0.5 μg of luciferase plasmid bearing _cPLA2_α gene promoter by lipofection, incubated for 6 h and then infected with adenovirus carrying GFP-Sp1 (Ad-GFP-Sp1) at 50 MOI. Cells infected with Ad-GFP were used as control. The luciferase activities and protein concentrations were determined. The results shown represent the means ± SEM of three determinations. Cell lysates were prepared and DNA affinity precipitation assay was performed as described under Materials and Methods section. Binding of Sp1 and phospho-c-Jun (Ser73) to cPLA2α Sp1 probes was analyzed by western blot.
Figure 3.
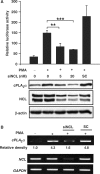
Effect of c-Jun on PMA-induced expression of cPLA2α in A549 cells. (A) Cells were transfected with c-Jun siRNA oligonucleotide by lipofection, incubated for 6 h and then transfected with 0.5 μg of pPLA 599. Cells were incubated for 24 h and then treated with 5 nM PMA for 18 h. The luciferase activities and protein concentrations were determined and normalized. The results shown represent the means ± SEM of three determinations. Expressions of c-Jun, cPLA2α and β-actin proteins were analyzed by western blot analysis using anti-c-Jun, cPLA2α and β-actin antibodies, respectively. SC: scramble oligonucleotides. Statistical significance (***P < 0.001) between PMA with c-Jun siRNA and PMA alone was analyzed by Student's _t_-test. (B) Cells were transfected with 0.5 μg of pRSVjun expression vector and 0.5 μg of luciferase plasmid bearing _cPLA2_α gene promoter by lipofection, incubated further for 36 h and the luciferase activities and protein concentrations were determined. The expression ratio of pRSVjun treated to control cells is indicated. The results shown represent the means ± SEM of 3 to 7 independent experiments in triplicate wells for each construct.
Figure 4.
PMA induces the complex formation and the binding of c-Jun, nucleolin and Sp1 to _cPLA2_α gene promoter in A549 cells. (A) Cells were starved for 18 h in serum-free culture medium and then treated with 5 nM PMA for a different time period as indicated. The Sp1, nucleolin (NCL) and c-Jun proteins were detected by anti-Sp1, anti-nucleolin and anti-c-Jun antibodies, respectively. (B and C) Nuclear extracts were immunoprecipitated (IP) with antibodies (Ab) against Sp1 and c-Jun. The proteins were subjected to SDS–PAGE and analyzed by western blotting with antibodies against c-Jun, NCL and Sp1. IgG: negative control of antibodies. (D and E) Cells were starved for 18 h in serum-free culture medium and then treated with 5 nM PMA for a different time period as indicated. Nuclear extracts were prepared and DNA affinity precipitation assay was performed as described under Materials and Methods section. Binding of Sp1, NCL, c-Jun and phospho-c-Jun (Ser73) to Sp1 probes were analyzed by western blot. The streptavidin-agarose beads were used to serve as a nonspecific binding control. (F) Cross-linked chromatin derived from PMA-treated cells was immunoprecipitated with c-Jun, NCL and Sp1 antibodies and analyzed by PCR with specific primers for the region from −239 to +19 bp of cPLA2α promoter. Input: nonimmunoprecipitated cross-linked chromatin.
Figure 5.
Effect of nucleolin on PMA-induced expression of cPLA2α in A549 cells. (A) Cells were transfected with nucleolin siRNA oligonucleotide (siNCL) by lipofection, incubated for 6 h and then transfected with 0.5 μg of pPLA 599 again. Cells were incubated for 24 h and then treated with 5 nM PMA for 18 h. The luciferase activities and protein concentrations were determined and normalized. The results shown represent the means ± SEM of three determinations. Expressions of nucleolin, cPLA2α and β-actin proteins were analyzed by western blot analysis using anti-nucleolin, cPLA2α and β-actin antibodies, respectively. SC: scramble oligonucleotides. Statistical significance (**P < 0.01 and ***P < 0.001) between PMA with nucleolin siRNA and PMA alone was analyzed by Student's _t_-test. (B) Cells were transfected with nucleolin siRNA oligonucleotide by lipofection for 36 h and then treated with 5 nM PMA for 18 h. Total RNA was extracted for RT-PCR with nucleolin, cPLA2 and GAPDH primers. The relative density of PCR products was quantified as indicated. SC: scramble oligonucleotides.
Figure 6.
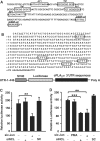
Effect of nucleolin on mRNA stability. (A) Putative transcription factor binding sites in the SV40 promoter of the pGL3-promoter vector were scanned by the computer program available on the Web site (//transfac.gbf.de/TRANSFAC/index.html). Each putative consensus sequence in the SV40 promoter is enclosed in a box. (B) Putative HuR-binding sites in cPLA2α 3′UTR are enclosed in boxes. The sequence of transcription stop site is underlined. Schematic diagram of UTR-1-446 vector was presented. (C and D) A549 Cells were transfected with nucleolin or c-Jun siRNA oligonucleotides by lipofection, incubated for 6 h and then transfected with 1 μg of pGL3 (C) and UTR-1-446 (D) vectors. Cells were incubated for 24 h and then the luciferase activities and protein concentrations were determined and normalized. The results shown represent the means ± SEM of three determinations. SC: scramble oligonucleotides. Statistical significance (**P < 0.01 and ***P < 0.001) between c-Jun siRNA and control or nucleolin siRNA and control was analyzed by Student's _t_-test.
Figure 7.
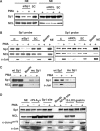
Effect of nucelolin and Sp1 on the recruitment of c-Jun to Sp1-dependent gene in A549 cells. (A and B) Cells were transfected with nucleolin and Sp1 siRNA oligonucleotides by lipofection, incubated for 30 h and then treated with 5 nM PMA for 3 h. Nuclear extracts (NE) were prepared and DNA affinity precipitation assay was performed as described under Materials and Methods section. Binding of Sp1, nucleolin and c-Jun to Sp1 probes were analyzed by western blot. The streptavidin-agarose beads were used to serve as a nonspecific binding control. SC: scramble oligonucleotides. (C and D) Cells were treated with 5 nM PMA for 3 h. Nuclear extracts (NE) were prepared and DNA affinity precipitation assay was performed as described under Materials and Methods section. Binding of Sp1, nucleolin, phospho-c-Jun (Ser73) and c-Jun to cPLA2α Sp1 (wt Sp1), cPLA2α Sp1 mutant (mu Sp1), 12(S)-lipoxygenase (12-LOX), gastrin, consensus Sp1 site (Sp1 site) and consensus NFκB site probes were analyzed by western blot. The streptavidin-agarose beads were used to serve as a nonspecific binding control.
Figure 8.
Nucelolin is essential for c-Jun/Sp1-regulated gene expression. (A and B) A431 cells were transfected with nucleolin siRNA oligonucleotides by lipofection, incubated for 6 h and then transfected with 0.5 μg of pXLO-7-1 (A) and pXC918 (B) vectors bearing the 12(S)-lipoxygenase and cyclooxygenase-2 promoters, respectively. Cells were incubated for 24 h and pXLO-7-1- and pXC918-transfected cells were treated with EGF (50 ng/ml) for 18 and 6 h, respectively. The luciferase activities and protein concentrations were determined and normalized. The results shown represent the means ± SEM of three determinations. SC: scramble oligonucleotides. Statistical significance (***P < 0.001) between EGF with nucleolin siRNA and EGF alone was analyzed by Student's _t_-test.
Similar articles
- Functional role of extracellular signal-regulated kinase activation and c-Jun induction in phorbol ester-induced promoter activation of human 12(S)-lipoxygenase gene.
Chen BK, Tsai TY, Huang HS, Chen LC, Chang WC, Tsai SB, Chang WC. Chen BK, et al. J Biomed Sci. 2002 Mar-Apr;9(2):156-65. doi: 10.1007/BF02256027. J Biomed Sci. 2002. PMID: 11914583 - PP2B-mediated dephosphorylation of c-Jun C terminus regulates phorbol ester-induced c-Jun/Sp1 interaction in A431 cells.
Chen BK, Huang CC, Chang WC, Chen YJ, Kikkawa U, Nakahama K, Morita I, Chang WC. Chen BK, et al. Mol Biol Cell. 2007 Mar;18(3):1118-27. doi: 10.1091/mbc.e06-09-0797. Epub 2007 Jan 10. Mol Biol Cell. 2007. PMID: 17215518 Free PMC article. - Activation of human monoamine oxidase B gene expression by a protein kinase C MAPK signal transduction pathway involves c-Jun and Egr-1.
Wong WK, Ou XM, Chen K, Shih JC. Wong WK, et al. J Biol Chem. 2002 Jun 21;277(25):22222-30. doi: 10.1074/jbc.M202844200. Epub 2002 Apr 15. J Biol Chem. 2002. PMID: 11956220 Free PMC article. - Cell signaling and gene regulation of human 12(S)-lipoxygenase expression.
Chang WC. Chang WC. Prostaglandins Other Lipid Mediat. 2003 Jul;71(3-4):277-85. doi: 10.1016/s1098-8823(03)00048-0. Prostaglandins Other Lipid Mediat. 2003. PMID: 14518567 Review. - Beyond biomarkers: Exploring the diverse potential of a novel phosphoprotein in lung cancer management.
Rostami A, Ranjbar E, Amiri S, Ezzatifar F. Rostami A, et al. J Cell Mol Med. 2024 Sep;28(18):e70077. doi: 10.1111/jcmm.70077. J Cell Mol Med. 2024. PMID: 39304978 Free PMC article. Review.
Cited by
- Interplay between REST and nucleolin transcription factors: a key mechanism in the overexpression of genes upon increased phosphorylation.
Tediose T, Kolev M, Sivasankar B, Brennan P, Morgan BP, Donev R. Tediose T, et al. Nucleic Acids Res. 2010 May;38(9):2799-812. doi: 10.1093/nar/gkq013. Epub 2010 Jan 25. Nucleic Acids Res. 2010. PMID: 20100803 Free PMC article. Retracted. - Cytosolic phospholipase A₂: physiological function and role in disease.
Leslie CC. Leslie CC. J Lipid Res. 2015 Aug;56(8):1386-402. doi: 10.1194/jlr.R057588. Epub 2015 Apr 2. J Lipid Res. 2015. PMID: 25838312 Free PMC article. Review. - Identification and characterization of nucleolin as a COUP-TFII coactivator of retinoic acid receptor β transcription in breast cancer cells.
Litchfield LM, Riggs KA, Hockenberry AM, Oliver LD, Barnhart KG, Cai J, Pierce WM Jr, Ivanova MM, Bates PJ, Appana SN, Datta S, Kulesza P, McBryan J, Young LS, Klinge CM. Litchfield LM, et al. PLoS One. 2012;7(5):e38278. doi: 10.1371/journal.pone.0038278. Epub 2012 May 31. PLoS One. 2012. PMID: 22693611 Free PMC article. - Blockage of Orai1-Nucleolin interaction meditated calcium influx attenuates breast cancer cells growth.
Gu C, Zhang W, Yang E, Gu C, Zhang Z, Ke J, Wang X, Wu S, Li S, Wu F. Gu C, et al. Oncogenesis. 2022 Sep 15;11(1):55. doi: 10.1038/s41389-022-00429-z. Oncogenesis. 2022. PMID: 36109490 Free PMC article.
References
- Cabral GA. Lipids as bioeffectors in the immune system. Life Sci. 2005;77:1699–1710. - PubMed
- Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Arachidonic acid cascade in endothelial pathobiology. Microvasc. Res. 2005;69:107–127. - PubMed
- Xu XC. COX-2 inhibitors in cancer treatment and prevention, a recent development. Anticancer Drugs. 2002;13:127–137. - PubMed
- Hubbard WC, Alley MC, McLemore TL, Boyd MR. Profiles of prostaglandin biosynthesis in sixteen established cell lines derived from human lung, colon, prostate, and ovarian tumors. Cancer Res. 1988;48:4770–4775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous