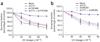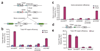A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair - PubMed (original) (raw)
. 2007 Dec;14(12):1165-72.
doi: 10.1038/nsmb1332. Epub 2007 Nov 18.
Yujiang Shi, Peter Mulligan, Frédérique Gay, Joseph Landry, Huifei Liu, Ju Lu, Hank H Qi, Weijia Wang, Jac A Nickoloff, Carl Wu, Yang Shi
Affiliations
- PMID: 18026119
- PMCID: PMC2754171
- DOI: 10.1038/nsmb1332
A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair
Su Wu et al. Nat Struct Mol Biol. 2007 Dec.
Abstract
DNA damage repair is crucial for the maintenance of genome integrity and cancer suppression. We found that loss of the mouse transcription factor YY1 resulted in polyploidy and chromatid aberrations, which are signatures of defects in homologous recombination. Further biochemical analyses identified a YY1 complex comprising components of the evolutionarily conserved INO80 chromatin-remodeling complex. Notably, RNA interference-mediated knockdown of YY1 and INO80 increased cellular sensitivity toward DNA-damaging agents. Functional assays revealed that both YY1 and INO80 are essential in homologous recombination-based DNA repair (HRR), which was further supported by the finding that YY1 preferentially bound a recombination-intermediate structure in vitro. Collectively, these observations reveal a link between YY1 and INO80 and roles for both in HRR, providing new insight into mechanisms that control the cellular response to genotoxic stress.
Figures
Figure 1
Loss of YY1 results in polyploidy and chromosome structural aberrations. (a) Metaphase spread from YY1f/f MEFs plus Cre, showing polyploidy. Wild-type and YY1f/f MEFs were transduced with Ad-Cre. (b) Metaphase spread from the YY1f/f MEFs plus Cre with multiple structural abnormalities (arrowheads). (c–e) Enlargements of the typical quadriradial structure (c), triradial structure (d) and chromatid break (e). (f) Percentages of aneuploid and polyploid chromosomes, which differ significantly between YY1f/f and YY1f/f plus Cre cultures. Difference between wild-type (WT) and YY1f/f plus Cre cultures was also statistically significant but is not shown. n indicates the number of metaphase spreads scored. (g) Percentages of abnormal chromosome structures, which differ significantly between YY1f/f and YY1f/f plus Cre cultures.
Figure 2
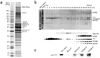
YY1 associates with mammalian Ino80 complex. (a) Silver staining of the YY1 complex separated on 4%–12% SDS-PAGE gel. The YY1 complex was immunopurified from nuclear extract prepared from HeLaS cells stably expressing Flag-HA-YY1. Subunits of INO80 complex are labeled in gray. (b) The double-purified YY1 complex in a was further fractionated by 10%–40% glycerol-gradient sedimentation. Fractions were visualized by silver staining (top) and immunoblotting with indicated antibodies (bottom). Input (3%) and molecular weight (MW) marker were also included on the silver-staining gel. (c) YY1 interacts with subunits of INO80 complex in vivo. Total HeLa cell lysates were immunoprecipitated with BAF53, TIP49A and TIP49B antibodies. YY1 levels in immunoprecipitates and 15% input were compared by western blotting with the YY1 antibody.
Figure 3
YY1 interacts with INO80 subunits in vitro and in vivo. (a) Co-immunoprecipitation between YY1 and INO80 upon DNA damage. HeLa cells transfected with Flag-INO80, HA-YY1 or both were radiated with UV (30 J M−2) and lysed 2 or 4 h after stress. Lysates were immunoprecipitated with Flag or HA affinity beads, probed with anti-Flag and reprobed with anti-HA. Lysate western blots show the protein levels of Flag-INO80 (v), HA-YY1 (vi), γH2AX (vii) and GAPDH (viii) in 10% input. IP, immunoprecipitation; IB, immunoblot. (b) Diagrams of human INO80 full-length protein and fragments used to map the YY1-binding domain. Abilities of indicated INO80 fragments to bind GST-YY1 protein are summarized on the right. (c,d) In vitro binding of indicated proteins to YY1 protein. Upper gels, amounts of 35S-labeled, _in vitro_–translated proteins retained on glutathione-agarose beads after pull-down assay, compared to the corresponding input (10%). Lower gels, Coomassie blue staining of GST and GST-YY1 proteins used for this experiment. In d, p53 and exportin 1 were used as positive and negative binding controls, respectively. (e) GST-YY1 pull-down assay with bacterially purified Flag-TIP49A and Flag-TIP49B. Top gel, pulled down samples analyzed by western blotting with the Flag antibody. Bottom gel, GST fusion proteins visualized by Coomassie blue staining.
Figure 4
Knockdown of TIP49B and INO80 leads to UV hypersensitivity. (a) U2OS cells were transfected with plasmids expressing shRNAs (sh) that target mGFP (negative control) (Supplementary Fig. 1g), YY1 or TIP49B, or both YY1 and TIP49B, for 48 h and treated with indicated doses of UV light. Survival fraction is the number of live cells after irradiation divided by the number of live cells after mock treatment and is expressed as a percentage. About 4,000 cells were counted in at least three independent experiments. Data are presented as means ± s.e.m. (b) As in a, cells were transfected with plasmids expressing shRNAs that target YY1, INO80 or both.
Figure 5
Impaired homology-directed repair of a chromosomal DSB in YY1- and INO80-deficient 293T and HT-1080 cell lines. (a) Schematic showing how, in the Neo direct-repeat substrate, I-SceI–induced DSBs can result in gene-conversion or deletion products. Neo direct repeats of 1.4 kilobases (open boxes) flank the Gpt gene. Transcription of the upstream Neo is driven by the TEAD1 (here called SV40) promoter and an I-SceI recognition site interrupts the reading frame, whereas the downstream Neo lacks a promoter. Shaded box, SV40 promoter; green arrow, functional Neo gene after repair. (b) Plasmid expressing shRNA constructs was cotransfected with I-SceI plasmid or control plasmid (SceI− plasmid; ‘None’ in key) into HR-293T cells. Total HRR frequencies were calculated as the number of G418-resistant colonies per viable cell plated in selective medium. Blue bars, spontaneous homologous recombination events; purple bars, I-SceI–induced events. Error bars indicate s.e.m. (c) Gene-conversion frequencies, calculated as the number of colonies resistant to both G418 and mycophenolic acid per viable cell plated. (d) Structure of inverted puromycin (Pac) repeats flanking the cytomegalovirus (CMV) Bsd promoter in the HT1080–1885 cell line. The upstream Pac is driven by the mouse Pgk promoter and inactivated by insertion of an I-SceI site in the coding sequence; the downstream donor Pac lacks a promoter. (e) Total HRR frequencies in Pac inverted-repeat HT1080–1885 strain. shRNA constructs were cotransfected with I-SceI plasmid or control plasmid into HT1080–1885 cells. Total HRR frequencies were calculated as the number of puromycin-resistant colonies per viable cell plated in selective medium. Error bars indicate s.e.m.
Figure 6
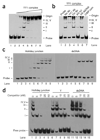
YY1 binds a recombination-intermediate structure in vitro. (a) Purified YY1 complex was incubated with biotinylated Holliday-junction DNA (probe), and the binding reactions were resolved on 4% nondenaturing PAGE gels. Reactions contained 10 nM probe and YY1 complex at 0, 10, 20, 40, 60, 80, 100 and 200 nM per reaction in lanes 1–8, respectively. The positions of unbound probe, gel origin and YY1 band-shift complexes I–V are indicated. (b) Specificity determination using YY1 supershift and unlabeled competitor DNA binding assays. Lane 1, probe only; lane 2, 40 nM YY1 complex; lane 3, supershift with YY1 antibody; lane 4, supershift with control antibody; lane 5, excess unlabeled Holliday junction (50 nM); lane 6, excess unlabeled dsDNA (50 nM); lane 7, CtBP complex. Reactions contained 10 nM probe. (c) Purified His-YY1 was incubated with biotinylated Holliday junction DNA (probe) or dsDNA probes, and the binding reactions were resolved on 4% nondenaturing PAGE gels. Reactions contained 1 nM probe and His-YY1 at 0, 100, 200, 400, 500, 600, 700, 800 and 900 nM in lanes 1–9, respectively. The positions of unbound probe and His-YY1 band-shift complexes I–V are indicated. (d) Specificity determination. Unlabeled DNA structures were titrated against biotinylated Holliday junction probe (1 nM). Lanes 1, 6 and 11 contain 600 nM His-YY1. The type of unlabeled competitor DNA is indicated above each of the three titration series (lanes 2–5, 7–10 and 12–15); concentrations of competitor DNA in each series were 5-fold, 10-fold, 25-fold and 50-fold molar excess.
Similar articles
- YY1 functions with INO80 to activate transcription.
Cai Y, Jin J, Yao T, Gottschalk AJ, Swanson SK, Wu S, Shi Y, Washburn MP, Florens L, Conaway RC, Conaway JW. Cai Y, et al. Nat Struct Mol Biol. 2007 Sep;14(9):872-4. doi: 10.1038/nsmb1276. Epub 2007 Aug 26. Nat Struct Mol Biol. 2007. PMID: 17721549 - Human INO80/YY1 chromatin remodeling complex transcriptionally regulates the BRCA2- and CDKN1A-interacting protein (BCCIP) in cells.
Su J, Sui Y, Ding J, Li F, Shen S, Yang Y, Lu Z, Wang F, Cao L, Liu X, Jin J, Cai Y. Su J, et al. Protein Cell. 2016 Oct;7(10):749-760. doi: 10.1007/s13238-016-0306-1. Epub 2016 Aug 17. Protein Cell. 2016. PMID: 27535137 Free PMC article. - An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update.
Banerjee S, Roy S. Banerjee S, et al. Cell Cycle. 2021 Sep;20(18):1760-1784. doi: 10.1080/15384101.2021.1966584. Epub 2021 Aug 26. Cell Cycle. 2021. PMID: 34437813 Free PMC article. Review. - Symmetric neural progenitor divisions require chromatin-mediated homologous recombination DNA repair by Ino80.
Keil JM, Doyle DZ, Qalieh A, Lam MM, Funk OH, Qalieh Y, Shi L, Mohan N, Sorel A, Kwan KY. Keil JM, et al. Nat Commun. 2020 Jul 31;11(1):3839. doi: 10.1038/s41467-020-17551-4. Nat Commun. 2020. PMID: 32737294 Free PMC article. - DNA helicases involved in DNA repair and their roles in cancer.
Brosh RM Jr. Brosh RM Jr. Nat Rev Cancer. 2013 Aug;13(8):542-58. doi: 10.1038/nrc3560. Epub 2013 Jul 11. Nat Rev Cancer. 2013. PMID: 23842644 Free PMC article. Review.
Cited by
- Cistrome analysis of YY1 uncovers a regulatory axis of YY1:BRD2/4-PFKP during tumorigenesis of advanced prostate cancer.
Xu C, Tsai YH, Galbo PM, Gong W, Storey AJ, Xu Y, Byrum SD, Xu L, Whang YE, Parker JS, Mackintosh SG, Edmondson RD, Tackett AJ, Huang J, Zheng D, Earp HS, Wang GG, Cai L. Xu C, et al. Nucleic Acids Res. 2021 May 21;49(9):4971-4988. doi: 10.1093/nar/gkab252. Nucleic Acids Res. 2021. PMID: 33849067 Free PMC article. - The oncogenic role of Yin Yang 1.
Zhang Q, Stovall DB, Inoue K, Sui G. Zhang Q, et al. Crit Rev Oncog. 2011;16(3-4):163-97. doi: 10.1615/critrevoncog.v16.i3-4.30. Crit Rev Oncog. 2011. PMID: 22248053 Free PMC article. Review. - Epigenetic regulation of genomic integrity.
Deem AK, Li X, Tyler JK. Deem AK, et al. Chromosoma. 2012 Apr;121(2):131-51. doi: 10.1007/s00412-011-0358-1. Epub 2012 Jan 17. Chromosoma. 2012. PMID: 22249206 Free PMC article. Review. - The Role of Ataxia Telangiectasia Mutant and Rad3-Related DNA Damage Response in Pathogenesis of Human Papillomavirus.
Luo Y, Hong S. Luo Y, et al. Pathogens. 2020 Jun 23;9(6):506. doi: 10.3390/pathogens9060506. Pathogens. 2020. PMID: 32585979 Free PMC article. Review. - Yin Yang 1 extends the Myc-related transcription factors network in embryonic stem cells.
Vella P, Barozzi I, Cuomo A, Bonaldi T, Pasini D. Vella P, et al. Nucleic Acids Res. 2012 Apr;40(8):3403-18. doi: 10.1093/nar/gkr1290. Epub 2011 Dec 30. Nucleic Acids Res. 2012. PMID: 22210892 Free PMC article.
References
- Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 2001;27:247–254. - PubMed
- van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2001;2:196–206. - PubMed
- Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat. Rev. Mol. Cell Biol. 2006;7:739–750. - PubMed
- Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21:8981–8993. - PubMed
- Moynahan ME. The cancer connection: BRCA1 and BRCA2 tumor suppression in mice and humans. Oncogene. 2002;21:8994–9007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases