Human tankyrases are aberrantly expressed in colon tumors and contain multiple epitopes that induce humoral and cellular immune responses in cancer patients - PubMed (original) (raw)
doi: 10.1007/s00262-007-0423-z. Epub 2007 Nov 20.
Inna N Lavrik, Julia Karbach, Svetlana V Khlgatian, Ekaterina P Koroleva, Pavel V Belousov, Kirill N Kashkin, Alexander Knuth, Elke Jager, Nai-Wen Chi, Dmitry V Kuprash, Sergei A Nedospasov
Affiliations
- PMID: 18026951
- PMCID: PMC11030928
- DOI: 10.1007/s00262-007-0423-z
Human tankyrases are aberrantly expressed in colon tumors and contain multiple epitopes that induce humoral and cellular immune responses in cancer patients
Yuriy V Shebzukhov et al. Cancer Immunol Immunother. 2008 Jun.
Abstract
Purpose: Tankyrases 1 and 2 are telomere-associated poly(ADP-ribose) polymerases (PARP) that can positively regulate telomere elongation and interact with multiple cellular proteins. Recent reports implicated tankyrases as tumor antigens and potential targets of anticancer treatment. We examined expression of tankyrases in colon tumors and immune response to these enzymes in patients with different types of cancer.
Methods: mRNA and protein expression was evaluated by quantitative real-time RT-PCR and Western blotting, respectively. Humoral immune response to recombinant tankyrases was investigated by modified enzyme-linked immunoassays. Cellular immune response was analysed by ELISPOT and (51)Cr release assays.
Results: We found that both mRNA and protein levels of tankyrase 2 (TNKL) are upregulated in colon tumors. In contrast, protein level of tankyrase 1 (TNKS) is downregulated, while mRNA level shows variable changes. More than a quarter of colon cancer patients develop humoral immune response to at least one of the two tankyrases. In this study we mapped common and unique B-cell epitopes located in different domains of the two proteins. Additionally, we present evidence for T-cell responses both to epitopes that are unique for TNKL and to those shared between TNKL and TNKS.
Conclusion: Our study favors a biomarker usage of antibody response to tankyrases. Spontaneous CD8(+) T-cell responses to these enzymes are rare and further investigation is needed to evaluate tankyrases as potential targets for cancer immunotherapy.
Figures
Fig. 1
Immunogenic human poly(ADP-ribose)polymerases. a Domain structure of tankyrases and PARP-1. Tankyrases: HPS histidine-proline-serine rich domain of TNKS, NTD N-terminal domain of TNKL, ANK ankyrin-repeat domain, SAM sterile α motif, PARP poly(ADP-ribose) polymerase domain [6]. PARP-1 F1 and F2 zinc fingers, NLS nuclear localization signal motif, AM auto modification domain, PARP poly(ADP-ribose)polymerase domain [10]. b Alignment of catalytic domains of human tankyrases and PARP-1
Fig. 2
Expression levels of human tankyrases in colon tumors. a Quantitative real-time RT-PCR. TNKS and TNKL mRNA levels ratios (tumor vs. normal tissue) in patients with different stages of colorectal cancer are shown. *P < 0.005 according Student’s t test for paired samples. b Western blotting. Upper rows Immunoblotting with anti-TNKS and anti-TNKL antibodies, N normal colon, T tumor tissue. AP (only for TNKL) affinity precipitation. −GST (negative control), _+_GST-IRAP. Lower rows Normalization of loading by silver staining (representative major protein bands of approx. molecular weight 70 kDa are shown). The results of one of two representative experiments are shown
Fig. 3
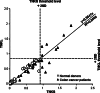
Serological cross-reactivity between TNKS and TNKL. Axes: relative (normalized to positive controls, see Table 2) intensities of serological reactions of tankyrases. Serological reactions to TNKS and TNKL demonstrate correlation designated as linear regression trend. Threshold levels (designated by dashed lines) separating serologically positive and negative samples: average value for sera from normal donors + 2 standard deviations
Fig. 4
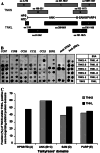
Sera of cancer patients recognize various protein domains of tankyrases. a Tankyrase fragments tested for serological reactivity. HPS histidine–proline–serine rich domain of TNKS, NTD N-terminal domain of TNKL, ANK ankyrin-repeat domain, SAM sterile α motif, PARP poly(ADP-ribose) polymerase domain [6]. TNKS-A to -E and TNKL-A to -E: tested tankyrases fragments. b Mini-array of recombinant protein domains and full-length tankyrases. Loading BSA (bovine serum albumin) −negative control, CC colon cancer patients, BC01 breast cancer patient. c Frequencies of humoral immune response to different tankyrases domains. Total of 38 pre-selected TNKL and/or TNKS positive sera tested (colon cancer19 patients, breast cancer 5 patients, ovarian cancer and head and neck cancer: in groups of 3 patients, lung cancer, renal cancer and melanoma: in groups of 2 patients, cervical cancer and prostate cancer: one patient)
Fig. 5
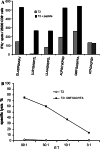
Cellular immune response to TNKL. a CD8+ reactivity to HLA-A2-restricted peptides in ELISPOT IFN-γ assay. TNKL peptides DLADPSAKAV (P2, aa 162–170), LLSYGADPTL (P8, aa 296–305), LLAHGADPTL (P23, aa 764–773), ALPSCYKPQV (P24, aa 802–811) and GMFGAGIYFA (P31, aa 1053–1062). ATADALFQV (C1): control irrelevant peptide. b Cytotoxicity test with CD8+ T cells from HLA-A2+ patient. Cytotoxicity was measured by 51Cr release assay
Similar articles
- Cloning and characterization of TNKL, a member of tankyrase gene family.
Kuimov AN, Kuprash DV, Petrov VN, Vdovichenko KK, Scanlan MJ, Jongeneel CV, Lagarkova MA, Nedospasov SA. Kuimov AN, et al. Genes Immun. 2001 Feb;2(1):52-5. doi: 10.1038/sj.gene.6363722. Genes Immun. 2001. PMID: 11294570 - Structural and functional analysis of parameters governing tankyrase-1 interaction with telomeric repeat-binding factor 1 and GDP-mannose 4,6-dehydratase.
Eisemann T, Langelier MF, Pascal JM. Eisemann T, et al. J Biol Chem. 2019 Oct 4;294(40):14574-14590. doi: 10.1074/jbc.RA119.009200. Epub 2019 Aug 2. J Biol Chem. 2019. PMID: 31375564 Free PMC article. - The Poly(ADP-ribose) Polymerase Enzyme Tankyrase Antagonizes Activity of the β-Catenin Destruction Complex through ADP-ribosylation of Axin and APC2.
Croy HE, Fuller CN, Giannotti J, Robinson P, Foley AVA, Yamulla RJ, Cosgriff S, Greaves BD, von Kleeck RA, An HH, Powers CM, Tran JK, Tocker AM, Jacob KD, Davis BK, Roberts DM. Croy HE, et al. J Biol Chem. 2016 Jun 10;291(24):12747-12760. doi: 10.1074/jbc.M115.705442. Epub 2016 Apr 11. J Biol Chem. 2016. PMID: 27068743 Free PMC article. - The telomeric PARP, tankyrases, as targets for cancer therapy.
Seimiya H. Seimiya H. Br J Cancer. 2006 Feb 13;94(3):341-5. doi: 10.1038/sj.bjc.6602951. Br J Cancer. 2006. PMID: 16421589 Free PMC article. Review. - Tankyrases as modulators of pro-tumoral functions: molecular insights and therapeutic opportunities.
Zamudio-Martinez E, Herrera-Campos AB, Muñoz A, Rodríguez-Vargas JM, Oliver FJ. Zamudio-Martinez E, et al. J Exp Clin Cancer Res. 2021 Apr 28;40(1):144. doi: 10.1186/s13046-021-01950-6. J Exp Clin Cancer Res. 2021. PMID: 33910596 Free PMC article. Review.
Cited by
- Tankyrases/β-catenin Signaling Pathway as an Anti-proliferation and Anti-metastatic Target in Hepatocarcinoma Cell Lines.
Huang J, Qu Q, Guo Y, Xiang Y, Feng D. Huang J, et al. J Cancer. 2020 Jan 1;11(2):432-440. doi: 10.7150/jca.30976. eCollection 2020. J Cancer. 2020. PMID: 31897238 Free PMC article. - The Anti-Tumor Activity of Succinyl Macrolactin A Is Mediated through the β-Catenin Destruction Complex via the Suppression of Tankyrase and PI3K/Akt.
Regmi SC, Park SY, Kim SJ, Banskota S, Shah S, Kim DH, Kim JA. Regmi SC, et al. PLoS One. 2015 Nov 6;10(11):e0141753. doi: 10.1371/journal.pone.0141753. eCollection 2015. PLoS One. 2015. PMID: 26544726 Free PMC article. - Regulatory roles of tankyrase 1 at telomeres and in DNA repair: suppression of T-SCE and stabilization of DNA-PKcs.
Dregalla RC, Zhou J, Idate RR, Battaglia CL, Liber HL, Bailey SM. Dregalla RC, et al. Aging (Albany NY). 2010 Oct;2(10):691-708. doi: 10.18632/aging.100210. Aging (Albany NY). 2010. PMID: 21037379 Free PMC article. - Regulation of Wnt/β-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding.
Mariotti L, Pollock K, Guettler S. Mariotti L, et al. Br J Pharmacol. 2017 Dec;174(24):4611-4636. doi: 10.1111/bph.14038. Epub 2017 Nov 5. Br J Pharmacol. 2017. PMID: 28910490 Free PMC article. Review. - RDX induces aberrant expression of microRNAs in mouse brain and liver.
Zhang B, Pan X. Zhang B, et al. Environ Health Perspect. 2009 Feb;117(2):231-40. doi: 10.1289/ehp.11841. Epub 2008 Sep 19. Environ Health Perspect. 2009. PMID: 19270793 Free PMC article.
References
- Chang P, Coughlin M, Mitchison TJ. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat Cell Biol. 2005;7:1133–1139. - PubMed
- Comtesse N, Zippel A, Walle S, Monz D, Backes C, Fischer U, Mayer J, Ludwig N, Hildebrandt A, Keller A, Steudel WI, Lenhof HP, Meese E. Complex humoral immune response against a benign tumor: Frequent antibody response against specific antigens as diagnostic targets. Proc Natl Acad Sci USA. 2005;102:9601–9606. doi: 10.1073/pnas.0500404102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials