An AMP nucleosidase gene knockout in Escherichia coli elevates intracellular ATP levels and increases cold tolerance - PubMed (original) (raw)
An AMP nucleosidase gene knockout in Escherichia coli elevates intracellular ATP levels and increases cold tolerance
Brittany A Morrison et al. Biol Lett. 2008.
Abstract
Disparate psychrophiles (e.g. glacier ice worms, bacteria, algae and fungi) elevate steady-state intracellular ATP levels as temperatures decline, which has been interpreted as a compensatory mechanism to offset reductions in molecular motion and Gibb's free energy of ATP hydrolysis. In this study, we sought to manipulate steady-state ATP levels in the mesophilic bacterium, Escherichia coli, to investigate the relationship between cold temperature survivability and elevated intracellular ATP. Based on known energetic pathways and feedback loops, we targeted the AMP nucleotidase (amn) gene, which is thought to encode the primary AMP degradative enzyme in prokaryotes. By knocking out amn in wild-type E. coli DY330 cells using recombineering methodology, we generated a mutant (AMNk) that elevated intracellular ATP levels by more than 30% across its viable temperature range. As temperature was lowered, the relative ATP disparity between AMNk and DY330 cells increased to approximately 66% at 10 degrees C, and was approximately 100% after storage at 0 degrees C for 5-7 days. AMNk cells stored at 0 degrees C for 7 days displayed approximately fivefold higher cell viability than wild-type DY330 cells treated in the same manner.
Figures
Figure 1
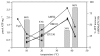
AMNk knockout cells elevated steady-state ATP levels compared with wild-type E. coli cells. ATP levels decreased in all mesophilic cells as temperature deviated from 37°C (line graph), but the relative difference in ATP levels between AMNk and DY330 increased (bars). The psychrophilic bacterium, _V_sp1, increased [ATP] as temperature decreased.
Figure 2
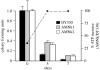
Survivability of AMNk versus DY330 cells after cold storage at 0°C. AMNk cells displayed an approximately 2.5- and approximately 5-fold increase (p<0.05) in cold survivability compared with DY330 after 5 and 7 days cold storage, respectively. Data are normalized and presented as a fraction of cells at zero time. ATP levels fell steadily in both AMNk and DY330 cells as a function of time, but were approximately 100% higher in AMNk after 5 days (0.99±0.02 versus 0.48±0.02 pmol ATP mg−1) and 7 days (0.85±0.01 versus 0.41±0.02 pmol ATP mg−1; dashed line, right axis; cf. figure 1).
Similar articles
- Structure and regulation of the AMP nucleosidase gene (amn) from Escherichia coli.
Leung HB, Kvalnes-Krick KL, Meyer SL, deRiel JK, Schramm VL. Leung HB, et al. Biochemistry. 1989 Oct 31;28(22):8726-33. doi: 10.1021/bi00448a008. Biochemistry. 1989. PMID: 2690948 - Manipulations of AMP metabolic genes increase growth rate and cold tolerance in Escherichia coli: implications for psychrophilic evolution.
Parry BR, Shain DH. Parry BR, et al. Mol Biol Evol. 2011 Jul;28(7):2139-45. doi: 10.1093/molbev/msr038. Epub 2011 Feb 7. Mol Biol Evol. 2011. PMID: 21300985 - [Energy charge changing by pathway manipulation of adenosine triphosphate metabolism in Escherichia coli].
Gu Z, Lv X, Zhang H. Gu Z, et al. Wei Sheng Wu Xue Bao. 2008 Jan;48(1):26-32. Wei Sheng Wu Xue Bao. 2008. PMID: 18338572 Chinese. - The regulatory subunit ε in Escherichia coli FOF1-ATP synthase.
Sielaff H, Duncan TM, Börsch M. Sielaff H, et al. Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):775-788. doi: 10.1016/j.bbabio.2018.06.013. Epub 2018 Jun 20. Biochim Biophys Acta Bioenerg. 2018. PMID: 29932911 Free PMC article. Review. - The mechanism and functions of ATP-dependent proteases in bacterial and animal cells.
Goldberg AL. Goldberg AL. Eur J Biochem. 1992 Jan 15;203(1-2):9-23. doi: 10.1111/j.1432-1033.1992.tb19822.x. Eur J Biochem. 1992. PMID: 1730246 Review. No abstract available.
Cited by
- ATP Content and Cell Viability as Indicators for Cryostress Across the Diversity of Life.
Bajerski F, Stock J, Hanf B, Darienko T, Heine-Dobbernack E, Lorenz M, Naujox L, Keller ERJ, Schumacher HM, Friedl T, Eberth S, Mock HP, Kniemeyer O, Overmann J. Bajerski F, et al. Front Physiol. 2018 Jul 17;9:921. doi: 10.3389/fphys.2018.00921. eCollection 2018. Front Physiol. 2018. PMID: 30065659 Free PMC article. - Structural Evolution of the Glacier Ice Worm Fo ATP Synthase Complex.
Lang SA, McIlroy P, Shain DH. Lang SA, et al. Protein J. 2020 Apr;39(2):152-159. doi: 10.1007/s10930-020-09889-x. Protein J. 2020. PMID: 32112190 - Enhancing D-pantothenate production in Escherichia coli through multiplex combinatorial strategies.
Huang L, Sui L, Yao Y, Ma Y, Zhou J, Zhang B, Liu Z, Zheng Y. Huang L, et al. Bioprocess Biosyst Eng. 2024 Nov 19. doi: 10.1007/s00449-024-03105-1. Online ahead of print. Bioprocess Biosyst Eng. 2024. PMID: 39560716 - A comparative UHPLC-Q/TOF-MS-based eco-metabolomics approach reveals temperature adaptation of four Nepenthes species.
Wong C, Ling YS, Wee JLS, Mujahid A, Müller M. Wong C, et al. Sci Rep. 2020 Dec 14;10(1):21861. doi: 10.1038/s41598-020-78873-3. Sci Rep. 2020. PMID: 33318532 Free PMC article. - Improved Preservation of Rat Small Intestine Transplantation Graft by Introduction of Mesenchymal Stem Cell-Secreted Fractions.
Teratani T, Fujimoto Y, Sakuma Y, Kasahara N, Maeda M, Miki A, Lefor AK, Sata N, Kitayama J. Teratani T, et al. Transpl Int. 2024 Jun 19;37:11336. doi: 10.3389/ti.2024.11336. eCollection 2024. Transpl Int. 2024. PMID: 38962471 Free PMC article.
References
- Ataullakhanov F.I, Vitvitsky V.M. What determines the intracellular ATP concentration. Biosci. Rep. 2002;22:501–511. doi:10.1023/A:1022069718709 - DOI - PubMed
- Atkinson D.E. Academic Press, Inc; New York, NY: 1977. Cellular energy metabolism and its regulation.
- Churchill T.A, Cheetham K.M, Simpkin S, Green C.J, Wang L.C.H, Fuller B.J. Liver metabolism in cold hypoxia: a comparison of energy metabolism and glycolysis in cold-sensitive and cold-resistant mammals. J. Comp. Physiol. B. 1994;164:396–404. doi:10.1007/BF00302556 - DOI - PubMed
- D'Amico S, Collins T, Marx J.C, Feller G, Gerday C. Activity–stability relationships in extremophilic enzymes. EMBO Rep. 2006;7:385–389. doi:10.1038/sj.embor.7400662 - DOI - PMC - PubMed
- Davies P.L, Baardsnes J, Kuiper M.J, Walker V.K. Structure and function of anti-freeze proteins. Phil. Trans. R. Soc. B. 2002;357:927–935. doi:10.1098/rstb.2002.1081 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials