Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice - PubMed (original) (raw)
Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice
Takuya Yokoe et al. J Physiol. 2008.
Abstract
Obstructive sleep apnoea (OSA) and type 2 diabetes frequently co-exist and potentially interact haemodynamically and metabolically. However, the confounding effects of obesity have obscured the examination of any independent or interactive effects of the hypoxic stress of OSA and the hyperglycaemia of type 2 diabetes on haemodynamic and metabolic outcomes. We have developed a chronically catheterized, unhandled, lean murine model to examine the effects of intermittent hypoxic (IH) exposure and exogenous glucose infusion on the diurnal pattern of arterial blood pressure and blood glucose, as well as pancreatic beta-cell growth and function. Four experimental groups of adult male C57BL/J mice were exposed to 80 h of (1) either IH (nadir of inspired oxygen 5-6% at 60 cycles h(-1) for 12 h during light period) or intermittent air (IA; control) and (2) continuous infusion of either 50% dextrose or saline (control). IH exposure during saline infusion caused a sustained increase in arterial blood pressure of 10 mmHg (P < 0.0001), reversed the normal diurnal rhythm of blood glucose (P < 0.03), doubled corticosterone levels (P < 0.0001), and increased replication of pancreatic beta-cells from 1.5 +/- 0.3 to 4.0 +/- 0.8% bromodeoxyuridine (BrdU)-positive) beta-cells. The combined stimulus of IH exposure and glucose infusion attenuated the hypertension, exacerbated the reversed diurnal glucose rhythm, and produced the highest rates of apoptosis in beta-cells, without any additive effects on beta-cell replication. We conclude that, in contrast to the development of sustained hypertension, IH impaired glucose homeostasis only during periods of hypoxic exposure. IH acted as a stimulus to pancreatic beta-cell replication, but the presence of hyperglycaemia may increase the hypoxic susceptibility of beta-cells. This model will provide a basis for future mechanistic studies as well as assessing the metabolic impact of common comorbities in OSA, including obesity, insulin resistance and type 2 diabetes.
Figures
Figure 1. Schematic of experimental protocol and time line for the study
Exposure to either intermittent hypoxia (IH) or intermittent air (IA) occurred during the 12 h of the light (L) period (all animals kept in constant room air during dark (D) period) with a simultaneous infusion of either 0.9% sodium chloride (saline infusion) or 50% dextrose (glucose infusion) containing 500 μg ml−1 bromodeoxyuridine (BrdU) at a constant rate of 100 μl h−1. *Arterial blood was sampled twice daily: immediately prior to lights on at 8 am and 10 h into the light period at 6 pm. For each blood sample a total of 100 μl whole blood was removed and 2 μl used to measure blood glucose, insulin and corticosterone. Plasma was extracted from the remaining blood and the red blood cells re-suspended and re-infused into the mouse.
Figure 2. Intermittent hypoxia caused a sustained hypertension that is attenuated in the presence of hyperglycaemia
A, changes in mean arterial blood pressure over time during saline infusion (intermittent hypoxia, n = 17; intermittent air n = 13; statistical difference determined by one-way ANOVA). B, changes in mean arterial blood pressure over time during glucose infusion (intermittent hypoxia, n = 14; intermittent air n = 14; statistical difference determined by one-way ANOVA). C, averaged changes in mean arterial blood pressure during the light and dark periods for all four experimental groups (note: data for dark period is plotted twice to highlight the diurnal rhythm). Analysis by two-way ANOVA revealed a significant effect of exposure (intermittent hypoxia > intermittent air; P < 0.0001) and infusion (glucose < saline; P < 0.0001), and a significant interaction (glucose infusion reduced the hypertensive effects of intermittent hypoxia exposure; P < 0.05).
Figure 3. Intermittent hypoxia reversed the normal diurnal glucose rhythm and caused a relative hypoglycaemia during the dark period
A, changes in blood glucose over time during saline infusion (intermittent hypoxia, n = 17; intermittent air n = 13). B, changes in blood glucose over time during glucose infusion (intermittent hypoxia, n = 14; intermittent air n = 14; statistical difference determined by one-way ANOVA). C, averaged changes in blood glucose during the light and dark periods for all four experimental groups (note: data for dark period is plotted twice to highlight the diurnal rhythm). Significant differences between the light and dark periods within an experimental group are marked to the right of the figure. Analysis by two-way ANOVA revealed a significant effect of exposure (intermittent hypoxia < intermittent air; _P_ < 0.0001) and infusion (glucose > saline; P < 0.0001), and a significant interaction (glucose infusion accentuated hypoglycaemia during intermittent hypoxia; P < 0.025).
Figure 4. Glucose infusion, but not intermittent hypoxia, increased plasma insulin levels
A, changes in plasma insulin over time during saline infusion (intermittent hypoxia, n = 17; intermittent air n = 13). B, changes in plasma insulin over time during glucose infusion (intermittent hypoxia, n = 14; intermittent air n = 14). C, averaged changes in plasma insulin during the light and dark periods for all four experimental groups (note: data for dark period is plotted twice to highlight the diurnal rhythm). Analysis by two-way ANOVA revealed a significant effect of infusion (glucose > saline; P < 0.0001), but no effect of intermittent hypoxia exposure and no interaction.
Figure 5. Intermittent hypoxia, but not glucose infusion, accentuated the light period spike in plasma corticosterone
A, changes in plasma corticosterone over time during saline infusion (intermittent hypoxia, n = 17; intermittent air n = 13; statistical difference determined by one-way ANOVA). B, changes in plasma corticosterone over time during glucose infusion (intermittent hypoxia, n = 14; intermittent air n = 14; statistical difference determined by one-way ANOVA). C, averaged changes in plasma corticosterone during the light and dark periods for all four experimental groups (note: data for dark period is plotted twice to highlight the diurnal rhythm). Significant differences between the light and dark periods within an experimental group are marked to the right of the figure. Analysis by two-way ANOVA revealed a significant effect of exposure (intermittent hypoxia > intermittent air; P < 0.0001), but no effect of glucose infusion and no interaction.
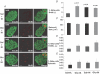
Figure 6. Glucose infusion and intermittent hypoxia increased pancreatic β-cell replication
A, representative islets stained for BrdU and insulin show glucose-induced and hypoxia-induced replication. Insets: high magnification confirms BrdU-positive nuclei belong to insulin-positive cells. Scale bars, 20 μm. B, mean percentage β-cell replication during intermittent air exposure (saline, n = 9; glucose n = 10) and intermittent hypoxia exposure (saline, n = 9; glucose n = 9). Statistical difference determined by one-way ANOVA and Dunnett's post hoc assessment relative to the saline + intermittent air control group are shown above bars. Analysis by two-way ANOVA revealed a significant effect of infusion (glucose > saline; P < 0.05), no effect of intermittent hypoxia, but a significant interaction (glucose infusion reduced the rate of replication due to intermittent hypoxia exposure; _P_ < 0.025). _C_, mean β-cell size during intermittent air exposure and intermittent hypoxia exposure (sample size same as _B_). _D_, mean changes in β-cells positive for TUNEL during intermittent air exposure and intermittent hypoxia exposure (sample size same as _B_). One-way ANOVA showed a trend for statistical significance (_P_ = 0.085) and Dunnett's _post hoc_ assessment showed an increase in apoptosis in the glucose + intermittent hypoxia group relative to the saline + intermittent air control group (_P_ = 0.032). Analysis by two-way ANOVA revealed a significant effect of exposure (intermittent hypoxia > intermittent air; P < 0.05), but no effect of glucose infusion and no interaction. E, mean percentage β-cells that were pyknotic during intermittent air exposure and intermittent hypoxia exposure (sample size same as B). Sal + IA, saline infusion and intermittent air exposure; Glu + IA, glucose infusion and intermittent air exposure; Sal + IH, saline infusion and intermittent hypoxia exposure; Glu + IH, glucose infusion and intermittent hypoxia exposure.
Similar articles
- Effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia in the frequently sampled intravenous glucose tolerance test.
Shin MK, Han W, Joo H, Bevans-Fonti S, Shiota M, Stefanovski D, Polotsky VY. Shin MK, et al. J Appl Physiol (1985). 2017 Apr 1;122(4):767-774. doi: 10.1152/japplphysiol.00975.2016. Epub 2017 Jan 19. J Appl Physiol (1985). 2017. PMID: 28104753 Free PMC article. - Impairment of pancreatic β-cell function by chronic intermittent hypoxia.
Wang N, Khan SA, Prabhakar NR, Nanduri J. Wang N, et al. Exp Physiol. 2013 Sep;98(9):1376-85. doi: 10.1113/expphysiol.2013.072454. Epub 2013 May 24. Exp Physiol. 2013. PMID: 23709585 Free PMC article. - Honokiol protects pancreatic β cell against high glucose and intermittent hypoxia-induced injury by activating Nrf2/ARE pathway in vitro and in vivo.
Li CG, Ni CL, Yang M, Tang YZ, Li Z, Zhu YJ, Jiang ZH, Sun B, Li CJ. Li CG, et al. Biomed Pharmacother. 2018 Jan;97:1229-1237. doi: 10.1016/j.biopha.2017.11.063. Epub 2017 Nov 13. Biomed Pharmacother. 2018. PMID: 29145148 - Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction.
Ryan S. Ryan S. J Physiol. 2017 Apr 15;595(8):2423-2430. doi: 10.1113/JP273312. Epub 2017 Jan 25. J Physiol. 2017. PMID: 27901270 Free PMC article. Review. - Relationship Between Intermittent Hypoxia and Type 2 Diabetes in Sleep Apnea Syndrome.
Ota H, Fujita Y, Yamauchi M, Muro S, Kimura H, Takasawa S. Ota H, et al. Int J Mol Sci. 2019 Sep 25;20(19):4756. doi: 10.3390/ijms20194756. Int J Mol Sci. 2019. PMID: 31557884 Free PMC article. Review.
Cited by
- Obstructive sleep apnea and type 2 diabetes: is there a link?
Pamidi S, Tasali E. Pamidi S, et al. Front Neurol. 2012 Aug 13;3:126. doi: 10.3389/fneur.2012.00126. eCollection 2012. Front Neurol. 2012. PMID: 23015803 Free PMC article. - Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes.
Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Reinke C, et al. J Appl Physiol (1985). 2011 Sep;111(3):881-90. doi: 10.1152/japplphysiol.00492.2011. Epub 2011 Jul 7. J Appl Physiol (1985). 2011. PMID: 21737828 Free PMC article. - Effect of Intermittent Hypoxia and Rimonabant on Glucose Metabolism in Rats: Involvement of Expression of GLUT4 in Skeletal Muscle.
Wang X, Yu Q, Yue H, Zeng S, Cui F. Wang X, et al. Med Sci Monit. 2015 Oct 27;21:3252-60. doi: 10.12659/msm.896039. Med Sci Monit. 2015. PMID: 26503060 Free PMC article. - Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice.
Alonso LC, Watanabe Y, Stefanovski D, Lee EJ, Singamsetty S, Romano LC, Zou B, Garcia-Ocaña A, Bergman RN, O'Donnell CP. Alonso LC, et al. Obesity (Silver Spring). 2012 Jul;20(7):1403-12. doi: 10.1038/oby.2012.36. Epub 2012 Feb 14. Obesity (Silver Spring). 2012. PMID: 22331130 Free PMC article. - Glucoregulatory consequences and cardiorespiratory parameters in rats exposed to chronic-intermittent hypoxia: effects of the duration of exposure and losartan.
Fenik VB, Singletary T, Branconi JL, Davies RO, Kubin L. Fenik VB, et al. Front Neurol. 2012 Apr 9;3:51. doi: 10.3389/fneur.2012.00051. eCollection 2012. Front Neurol. 2012. PMID: 22509173 Free PMC article.
References
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. - PubMed
- Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol. 2005;99:2028–2035. - PubMed
- Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical