Mechanisms controlling cell cycle exit upon terminal differentiation - PubMed (original) (raw)
Review
Mechanisms controlling cell cycle exit upon terminal differentiation
Laura A Buttitta et al. Curr Opin Cell Biol. 2007 Dec.
Abstract
Coordinating terminal differentiation with permanent exit from the cell cycle is crucial for proper organogenesis, yet how the cell cycle is blocked in differentiated tissues remains unclear. Important roles for retinoblastoma family proteins and Cyclin-dependent kinase inhibitors have been delineated, but in many cases it remains unclear what triggers cell cycle exit. This review focuses on describing recent advances in deciphering how terminal differentiation and exit from the cell cycle are coordinated.
Figures
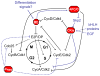
Figure 1. Molecular stop signs: Multiple mechanisms prohibit cell cycle progression upon terminal differentiation
Negative regulators of the cell cycle act as blockades, preventing proliferation upon terminal differentiation. Recent research has identified new signals impinging on known blockades such as the retinoblastoma proteins (Rbs) and Cyclin dependent kinase inhibitors (CKIs), as well as new negative regulators such as Prospero-like homeobox transcrption factors (Pros), and a new post-mitotic role for the Anaphase Promoting Complex/Cyclosome (APC/C). Regulation can be at the level of transcription (dotted lines) or post-transcriptionally (solid lines). Recently identified pathways highlighted in blue are discussed in this review.
Figure 2. Terminal differentiation and cell cycle exit are separable
Although terminal differentiation and cell cycle exit are coordinated, in some contexts they are separable. A. For example a neuron in the late Drosophila retina can continue cycling and undergo mitosis (indicated by phosphohistone H3 in red) while maintaining characteristics of terminal differentiation, such as expression of Elav (in blue) and projection of an axon (cell membrane in green, indicated by an arrow) if both E2F and Cyclin/Cdk activities are kept high [25]. B. Hair cells of the mouse inner ear lacking Rb expression also continue cycling upon terminal differentiation, providing another example where differentiation and cell cycle exit are separable. Myo7A expression in red labels hair cells of the cochlea, while BrdU in green labels cells in S-phase. Co-labeled cells differentiate but continue into S-phase (arrow) C. Recorded transduction currents in Rb−/− hair cells demonstrate mechanosensitivity [B and C adapted from 62].
Similar articles
- A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila.
Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA. Buttitta LA, et al. Dev Cell. 2007 Apr;12(4):631-43. doi: 10.1016/j.devcel.2007.02.020. Dev Cell. 2007. PMID: 17419999 - Molecular Competition in G1 Controls When Cells Simultaneously Commit to Terminally Differentiate and Exit the Cell Cycle.
Zhao ML, Rabiee A, Kovary KM, Bahrami-Nejad Z, Taylor B, Teruel MN. Zhao ML, et al. Cell Rep. 2020 Jun 16;31(11):107769. doi: 10.1016/j.celrep.2020.107769. Cell Rep. 2020. PMID: 32553172 Free PMC article. - Cell cycle exit upon myogenic differentiation.
Walsh K, Perlman H. Walsh K, et al. Curr Opin Genet Dev. 1997 Oct;7(5):597-602. doi: 10.1016/s0959-437x(97)80005-6. Curr Opin Genet Dev. 1997. PMID: 9388774 Review. - Coordination of cell cycle exit and differentiation of neuronal progenitors.
Politis PK, Thomaidou D, Matsas R. Politis PK, et al. Cell Cycle. 2008 Mar 15;7(6):691-7. doi: 10.4161/cc.7.6.5550. Epub 2008 Jan 1. Cell Cycle. 2008. PMID: 18239460 Review. - Beyond the cell cycle: a new role for Cdk6 in differentiation.
Grossel MJ, Hinds PW. Grossel MJ, et al. J Cell Biochem. 2006 Feb 15;97(3):485-93. doi: 10.1002/jcb.20712. J Cell Biochem. 2006. PMID: 16294322 Review.
Cited by
- Podocyte mitosis - a catastrophe.
Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P. Lasagni L, et al. Curr Mol Med. 2013 Jan;13(1):13-23. doi: 10.2174/1566524011307010013. Curr Mol Med. 2013. PMID: 23176147 Free PMC article. Review. - Arabidopsis RETINOBLASTOMA-RELATED is required for stem cell maintenance, cell differentiation, and lateral organ production.
Borghi L, Gutzat R, Fütterer J, Laizet Y, Hennig L, Gruissem W. Borghi L, et al. Plant Cell. 2010 Jun;22(6):1792-811. doi: 10.1105/tpc.110.074591. Epub 2010 Jun 4. Plant Cell. 2010. PMID: 20525851 Free PMC article. - Uniparental inheritance of cpDNA and the genetic control of sexual differentiation in Chlamydomonas reinhardtii.
Nishimura Y. Nishimura Y. J Plant Res. 2010 Mar;123(2):149-62. doi: 10.1007/s10265-009-0292-y. J Plant Res. 2010. PMID: 20196233 Review. - "Social Life" of Senescent Cells: What Is SASP and Why Study It?
Borodkina AV, Deryabin PI, Giukova AA, Nikolsky NN. Borodkina AV, et al. Acta Naturae. 2018 Jan-Mar;10(1):4-14. Acta Naturae. 2018. PMID: 29713514 Free PMC article. - Invasive Cell Fate Requires G1 Cell-Cycle Arrest and Histone Deacetylase-Mediated Changes in Gene Expression.
Matus DQ, Lohmer LL, Kelley LC, Schindler AJ, Kohrman AQ, Barkoulas M, Zhang W, Chi Q, Sherwood DR. Matus DQ, et al. Dev Cell. 2015 Oct 26;35(2):162-74. doi: 10.1016/j.devcel.2015.10.002. Dev Cell. 2015. PMID: 26506306 Free PMC article.
References
- Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene. 2000;247:1–15. - PubMed
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. - PubMed
- Koreth J, van den Heuvel S. Cell-cycle control in Caenorhabditis elegans: how the worm moves from G1 to S. Oncogene. 2005;24:2756–2764. - PubMed
- Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 GM070887/GM/NIGMS NIH HHS/United States
- U54 CA132383/CA/NCI NIH HHS/United States
- R01 GM070887-03/GM/NIGMS NIH HHS/United States
- U54 CA132381/CA/NCI NIH HHS/United States
- GM070887/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources