The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner - PubMed (original) (raw)
The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner
Didier Vertommen et al. Mol Microbiol. 2008 Jan.
Abstract
In Escherichia coli, DsbA introduces disulphide bonds into secreted proteins. DsbA is recycled by DsbB, which generates disulphides from quinone reduction. DsbA is not known to have any proofreading activity and can form incorrect disulphides in proteins with multiple cysteines. These incorrect disulphides are thought to be corrected by a protein disulphide isomerase, DsbC, which is kept in the reduced and active configuration by DsbD. The DsbC/DsbD isomerization pathway is considered to be isolated from the DsbA/DsbB pathway. We show that the DsbC and DsbA pathways are more intimately connected than previously thought. dsbA(-)dsbC(-) mutants have a number of phenotypes not exhibited by either dsbA(-), dsbC(-) or dsbA(-)dsbD(-) mutations: they exhibit an increased permeability of the outer membrane, are resistant to the lambdoid phage Phi80, and are unable to assemble the maltoporin LamB. Using differential two-dimensional liquid chromatographic tandem mass spectrometry/mass spectrometry analysis, we estimated the abundance of about 130 secreted proteins in various dsb(-) strains. dsbA(-)dsbC(-) mutants exhibit unique changes at the protein level that are not exhibited by dsbA(-)dsbD(-) mutants. Our data indicate that DsbC can assist DsbA in a DsbD-independent manner to oxidatively fold envelope proteins. The view that DsbC's function is limited to the disulphide isomerization pathway should therefore be reinterpreted.
Figures
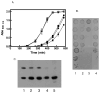
Fig. 1. The absence of dsbA and dsbC has phenotypical consequences
A. Growth curves of wild-type (□), _dsbC_− (▴), _dsbA_− (*), _dsbA_−_dsbD_− ( ) and _dsbA_−_dsbC_− (●) strains in M63 minimal media at 37°C. Growth was monitored at A600. B. SDS sensitivity of wild-type (lane 1), _dsbA_− (lane 2), _dsbA_−_dsbC_− (lane 3) and _dsbA_−_dsbD_− (lane 4) strains. Strains were grown in LB at 37°C to an A600 of 0.5. The cultures were then serially diluted 107-fold in 10-fold increments. 10 μl of each dilution were then spotted on LB plates containing 2.5% SDS and grown overnight. C. Western blot showing protein expression levels. The upper bands correspond to the LamB protein. Outer membrane proteins prepared from wild-type (lane 1), _dsbA_− (lane 2), _dsbC_− (lane 3), _dsbA_−_dsbC_− (lane 4), and _LamB_− (lane 5) strains. The lower bands correspond to an unknown protein recognized by the anti-LamB antibody, which was used as an internal standard.
) and _dsbA_−_dsbC_− (●) strains in M63 minimal media at 37°C. Growth was monitored at A600. B. SDS sensitivity of wild-type (lane 1), _dsbA_− (lane 2), _dsbA_−_dsbC_− (lane 3) and _dsbA_−_dsbD_− (lane 4) strains. Strains were grown in LB at 37°C to an A600 of 0.5. The cultures were then serially diluted 107-fold in 10-fold increments. 10 μl of each dilution were then spotted on LB plates containing 2.5% SDS and grown overnight. C. Western blot showing protein expression levels. The upper bands correspond to the LamB protein. Outer membrane proteins prepared from wild-type (lane 1), _dsbA_− (lane 2), _dsbC_− (lane 3), _dsbA_−_dsbC_− (lane 4), and _LamB_− (lane 5) strains. The lower bands correspond to an unknown protein recognized by the anti-LamB antibody, which was used as an internal standard.
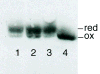
Fig. 2
In vivo redox state of DsbC. Exponentially growing cells (in LB) were TCA-precipitated, free cysteines were modified by AMS, and DsbC was detected by Western blot analysis. Lanes: 1, wild-type; 2, _dsb_A−; 3, _dsbA_−_dsbD_−; 4, _dsbD_−.
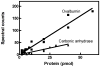
Fig. 3
The number of spectral counts correlates with the abundance of a protein. Varying amounts (2–60 pmoles) of two eukaryotic proteins, ovalbumin and carbonic anhydrase, were added to 300 μg of periplasmic proteins. The SC values obtained for these two proteins in the various samples were then plotted against the corresponding protein amounts. After linear regression, we found that the R2 values obtained for the spectral counts were 0.94 and 0.92 for ovalbumin and carbonic anhydrase, respectively. This indicates that the number of spectral counts reliably reflects protein abundance in the sample.
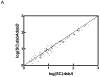
Fig. 4. The overall protein content of a _dsbA_−_dsbC_− mutant is different compared to _dsbA_− and _dsbA_−_dsbD_− strains
A. The logarithms of the SC values reported for the _dsbA_− strain were plotted against those reported for the _dsbA_−_dsbD_− mutant. Most of the SC values are similar in both strains, which is reflected by a quasi-linear distribution. B. The logarithms of the SC values reported for the _dsbA_− strain were plotted against those reported for the _dsbA_−_dsbC_− mutant. The distribution is more dispersed, which indicates that the overall protein content of this double mutant is different.
Fig. 4. The overall protein content of a _dsbA_−_dsbC_− mutant is different compared to _dsbA_− and _dsbA_−_dsbD_− strains
A. The logarithms of the SC values reported for the _dsbA_− strain were plotted against those reported for the _dsbA_−_dsbD_− mutant. Most of the SC values are similar in both strains, which is reflected by a quasi-linear distribution. B. The logarithms of the SC values reported for the _dsbA_− strain were plotted against those reported for the _dsbA_−_dsbC_− mutant. The distribution is more dispersed, which indicates that the overall protein content of this double mutant is different.
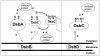
Fig. 5. A revised model for the formation of disulfide bonds in the E. coli periplasm
Disulfide bonds are introduced by the DsbA/DsbB pathway. Non-native disulfides are corrected by DsbC, which is recycled by DsbD. Both pathways are kinetically isolated. Our results indicate that DsbC is also able to function on the other side of the barrier where it assists DsbA in a DsbD-independent manner. DsbC may be acting as a stand-alone protein folding catalyst that cycles from the reduced to the oxidized state upon substrate oxidation and substrate reduction, respectively. Although kinetics data showed that DsbC is not a good substrate for DsbB, we cannot exclude that a slow oxidation of DsbC by DsbB may play a more significant role in the absence of DsbA. The Western blot data presented in Figure 2 also suggest that in the absence of DsbD, DsbA may be responsible for the oxidation of DsbC. The redox potentials of DsbA and DsbC are −125 mV and −130 mV, respectively.
Similar articles
- Mutants in DsbB that appear to redirect oxidation through the disulfide isomerization pathway.
Pan JL, Sliskovic I, Bardwell JC. Pan JL, et al. J Mol Biol. 2008 Apr 11;377(5):1433-42. doi: 10.1016/j.jmb.2008.01.058. Epub 2008 Jan 31. J Mol Biol. 2008. PMID: 18325532 Free PMC article. - Engineered DsbC chimeras catalyze both protein oxidation and disulfide-bond isomerization in Escherichia coli: Reconciling two competing pathways.
Segatori L, Paukstelis PJ, Gilbert HF, Georgiou G. Segatori L, et al. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10018-23. doi: 10.1073/pnas.0403003101. Epub 2004 Jun 25. Proc Natl Acad Sci U S A. 2004. PMID: 15220477 Free PMC article. - Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA.
Bader MW, Hiniker A, Regeimbal J, Goldstone D, Haebel PW, Riemer J, Metcalf P, Bardwell JC. Bader MW, et al. EMBO J. 2001 Apr 2;20(7):1555-62. doi: 10.1093/emboj/20.7.1555. EMBO J. 2001. PMID: 11285220 Free PMC article. - Protein Disulfide Exchange by the Intramembrane Enzymes DsbB, DsbD, and CcdA.
Bushweller JH. Bushweller JH. J Mol Biol. 2020 Aug 21;432(18):5091-5103. doi: 10.1016/j.jmb.2020.04.008. Epub 2020 Apr 16. J Mol Biol. 2020. PMID: 32305461 Free PMC article. Review. - Oxidative protein folding in bacteria.
Collet JF, Bardwell JC. Collet JF, et al. Mol Microbiol. 2002 Apr;44(1):1-8. doi: 10.1046/j.1365-2958.2002.02851.x. Mol Microbiol. 2002. PMID: 11967064 Review.
Cited by
- Making a chink in their armor: Current and next-generation antimicrobial strategies against the bacterial cell envelope.
Kadeřábková N, Mahmood AJS, Furniss RCD, Mavridou DAI. Kadeřábková N, et al. Adv Microb Physiol. 2023;83:221-307. doi: 10.1016/bs.ampbs.2023.05.003. Epub 2023 Jun 27. Adv Microb Physiol. 2023. PMID: 37507160 Free PMC article. - Distinct enzymatic strategies for de novo generation of disulfide bonds in membranes.
Li W. Li W. Crit Rev Biochem Mol Biol. 2023 Feb;58(1):36-49. doi: 10.1080/10409238.2023.2201404. Epub 2023 Apr 25. Crit Rev Biochem Mol Biol. 2023. PMID: 37098102 Free PMC article. - Breaking antimicrobial resistance by disrupting extracytoplasmic protein folding.
Furniss RCD, Kaderabkova N, Barker D, Bernal P, Maslova E, Antwi AAA, McNeil HE, Pugh HL, Dortet L, Blair JMA, Larrouy-Maumus G, McCarthy RR, Gonzalez D, Mavridou DAI. Furniss RCD, et al. Elife. 2022 Jan 13;11:e57974. doi: 10.7554/eLife.57974. Elife. 2022. PMID: 35025730 Free PMC article. - Structural characterization of PaFkbA: A periplasmic chaperone from Pseudomonas aeruginosa.
Huang Q, Yang J, Li C, Song Y, Zhu Y, Zhao N, Mou X, Tang X, Luo G, Tong A, Sun B, Tang H, Li H, Bai L, Bao R. Huang Q, et al. Comput Struct Biotechnol J. 2021 Apr 25;19:2460-2467. doi: 10.1016/j.csbj.2021.04.045. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34025936 Free PMC article. - How the assembly and protection of the bacterial cell envelope depend on cysteine residues.
Collet JF, Cho SH, Iorga BI, Goemans CV. Collet JF, et al. J Biol Chem. 2020 Aug 21;295(34):11984-11994. doi: 10.1074/jbc.REV120.011201. Epub 2020 Jun 2. J Biol Chem. 2020. PMID: 32487747 Free PMC article. Review.
References
- Altermark B, Smalas AO, Willassen NP, Helland R. The structure of Vibrio cholerae extracellular endonuclease I reveals the presence of a buried chloride ion. Acta Crystallogr D Biol Crystallogr. 2006;62:1387–1391. - PubMed
- Andersen CL, Matthey-Dupraz A, Missiakas D, Raina S. A new Escherichia coli gene, dsbG, encodes a periplasmic protein involved in disulphide bond formation, required for recycling DsbA/DsbB and DsbC redox proteins. Mol Microbiol. 1997;26:121–132. - PubMed
- Bader M, Muse W, Ballou DP, Gassner C, Bardwell JC. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. - PubMed
- Bardwell JC, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM057039/GM/NIGMS NIH HHS/United States
- R01 GM057039-09/GM/NIGMS NIH HHS/United States
- R01 GM064662/GM/NIGMS NIH HHS/United States
- R01 GM064662-04/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases