Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction - PubMed (original) (raw)
Review
. 2008 Mar;153 Suppl 1(Suppl 1):S298-309.
doi: 10.1038/sj.bjp.0707508. Epub 2007 Nov 26.
Affiliations
- PMID: 18037927
- PMCID: PMC2268080
- DOI: 10.1038/sj.bjp.0707508
Review
Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction
K Defea. Br J Pharmacol. 2008 Mar.
Abstract
G-protein-coupled receptors (GPCRs), also known as seven transmembrane receptors (7-TMRs), are the largest protein receptor superfamily in the body. These receptors and their ligands direct a diverse array of physiological responses, and hence have broad relevance to numerous diseases. As a result, they have generated considerable interest in the pharmaceutical industry as drug targets. Recently, GPCRs have been demonstrated to elicit signals through interaction with the scaffolding proteins, beta-arrestins-1 and 2, independent of heterotrimeric G-protein coupling. This review discusses several known G-protein-independent, beta-arrestin-dependent pathways and their potential physiological and pharmacological significance. The emergence of G-protein-independent signalling changes the way in which GPCR signalling is evaluated, from a cell biological to a pharmaceutical perspective and raises the possibility for the development of pathway specific therapeutics.
Figures
Figure 1
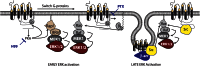
Types of β-arrestin-dependent, G-protein-independent signals. (a) Differential localization of ERK1/2. β-Arrestins can activate ERK1/2 downstream of numerous GPCRs and effectively ‘steal' the kinases away the G-protein-dependent pathway. In the case of PAR-2 and AT1R, signalling through Gαq leads to mobilization of intracellular Ca2+, activation of conventional PKCs and Ras-dependent activation of the MAPK module (Raf-1, MEK1/2 and ERK1/2). The activated ERKs translocate to the nucleus where they phosphorylate transcription factors (TF) leading to gene expression and proliferation. When activated through β-arrestins, the entire MAPK module is scaffolded onto the β-arrestin-bound receptor, forming an ‘endosomal scaffold' that promotes prolonged activation of ERK1/2 at the membrane or within the cytosol preventing the transcriptional and proliferative effects and promoting cytoskeletal reorganization and chemotaxis. (b) Opposing signalling: PAR-2 to PI3K. PAR-2 can both activate and inhibit PI3K in a cell-type-specific fashion, depending on the expression level of β-arrestins. Activation of the classical Gaq/Ca2+ pathway leads to increased activity of the p110 catalytic subunit of PI3K, leading to generation of PIP3. Recruitment of β-arrestins leads to the formation of a unique endosomal scaffold containing the regulatory (p85) and catalytic PI3K subunits. Binding to β-arrestins directly inhibits PI3K activity. (c) Synergistic signalling: AT1R and RhoA. AT1R activates RhoA, leading to stress fibre formation, through activation of the RhoA effector ROCK. Both β-arrestin-dependent recruitment to the receptor and coupling to Gαq are required for RhoA activation and subsequent stress fibre formation, indicating a convergence of the two pathways at the level of RhoA. AT1R, type I angiotensin II receptor; GPCR, G-protein-coupled receptor; MAPK, mitogen-activated protein kinase; PAR, protease-activated receptor.
Figure 2
Model of ERK1/2 activation by β2AR: contribution of G-protein- and β-arrestin-dependent signals. β2AR promotes several temporally distinct phases of ERK1/2 activation, each dependent on a different signalling moiety. Activation of PKA through coupling to Gαs and subsequent cAMP generation leads to activation of the small GTPase Rap1, which activates B-raf. B-Raf can then activate MEK1 and ERK1/2. PKA also phosphorylates the receptor leading to Gαi coupling, the release of free active Gβγ subunits and activation of Ras. Both of these pathways are rapid and transient, within minutes of receptor activation. Recruitment of β-arrestin allows scaffolding and activation of Src and subsequent Ras-dependent ERK1/2 activation. At high ligand concentrations, Src can bind directly to the receptor, bypassing the requirement for β-arrestins in Src activation.
Figure 3
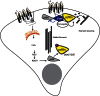
Localized activation and inactivation of cofilin by PAR-2. PAR-2 can activate cofilin, an actin filament-severing protein important for chemotaxis, through recruitment of β-arrestins and subsequent binding of cofilin, its activating phosphatase (chronophin) and its inhibitory kinase (LIMK). β-arrestin inhibits LIMK activity, preventing it from dephosphorylating and inactivating cofilin. The net result is increased cofilin activity at the leading edge, which is important for turnover of filaments during cell migration. Simultaneously, through coupling to Gαq, PAR-2 can activate LIMK, presumably in regions of the cell where filament stability is essential, leading to localized inhibition of cofilin. PAR, protease-activated receptor.
Similar articles
- Seven-transmembrane receptor signaling through beta-arrestin.
Shenoy SK, Lefkowitz RJ. Shenoy SK, et al. Sci STKE. 2005 Nov 1;2005(308):cm10. doi: 10.1126/stke.2005/308/cm10. Sci STKE. 2005. PMID: 16267056 Review. - Antidepressants, beta-arrestins and GRKs: from regulation of signal desensitization to intracellular multifunctional adaptor functions.
Golan M, Schreiber G, Avissar S. Golan M, et al. Curr Pharm Des. 2009;15(14):1699-708. doi: 10.2174/138161209788168038. Curr Pharm Des. 2009. PMID: 19442183 Review. - beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation.
Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. Tohgo A, et al. J Biol Chem. 2002 Mar 15;277(11):9429-36. doi: 10.1074/jbc.M106457200. Epub 2002 Jan 2. J Biol Chem. 2002. PMID: 11777902 - The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals.
Luttrell LM, Lefkowitz RJ. Luttrell LM, et al. J Cell Sci. 2002 Feb 1;115(Pt 3):455-65. doi: 10.1242/jcs.115.3.455. J Cell Sci. 2002. PMID: 11861753 Review.
Cited by
- Protease-Activated Receptors (PARs): Biology and Therapeutic Potential in Perioperative Stroke.
Mavridis T, Choratta T, Papadopoulou A, Sawafta A, Archontakis-Barakakis P, Laou E, Sakellakis M, Chalkias A. Mavridis T, et al. Transl Stroke Res. 2024 Feb 7. doi: 10.1007/s12975-024-01233-0. Online ahead of print. Transl Stroke Res. 2024. PMID: 38326662 Review. - Functional selectivity of EM-2 analogs at the mu-opioid receptor.
Piekielna-Ciesielska J, Malfacini D, Djeujo FM, Marconato C, Wtorek K, Calo' G, Janecka A. Piekielna-Ciesielska J, et al. Front Pharmacol. 2023 Feb 24;14:1133961. doi: 10.3389/fphar.2023.1133961. eCollection 2023. Front Pharmacol. 2023. PMID: 36909169 Free PMC article. - Dopamine, Immunity, and Disease.
Channer B, Matt SM, Nickoloff-Bybel EA, Pappa V, Agarwal Y, Wickman J, Gaskill PJ. Channer B, et al. Pharmacol Rev. 2023 Jan;75(1):62-158. doi: 10.1124/pharmrev.122.000618. Epub 2022 Dec 8. Pharmacol Rev. 2023. PMID: 36757901 Free PMC article. Review. - Identification and mechanism of G protein-biased ligands for chemokine receptor CCR1.
Shao Z, Shen Q, Yao B, Mao C, Chen LN, Zhang H, Shen DD, Zhang C, Li W, Du X, Li F, Ma H, Chen ZH, Xu HE, Ying S, Zhang Y, Shen H. Shao Z, et al. Nat Chem Biol. 2022 Mar;18(3):264-271. doi: 10.1038/s41589-021-00918-z. Epub 2021 Dec 23. Nat Chem Biol. 2022. PMID: 34949837 Free PMC article. - Functional Characterization of Spinocerebellar Ataxia Associated Dynorphin A Mutant Peptides.
Lieb A, Thaler G, Fogli B, Trovato O, Posch MA, Kaserer T, Zangrandi L. Lieb A, et al. Biomedicines. 2021 Dec 11;9(12):1882. doi: 10.3390/biomedicines9121882. Biomedicines. 2021. PMID: 34944698 Free PMC article.
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of {beta}-arrestin and G Protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. - PubMed
- Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjolbye AL, et al. The angiotensin type 1 receptor activates extracellular signal-regulated kinases 1 and 2 by G protein-dependent and -independent pathways in cardiac myocytes and langendorff-perfused hearts. Basic & Clinical Pharmacology & Toxicology. 2007;100:289–295. - PubMed
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. - PubMed
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt–GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials