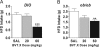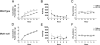Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors - PubMed (original) (raw)
Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors
Daniel D Lam et al. Endocrinology. 2008 Mar.
Abstract
The neurotransmitter serotonin (5-hydroxytryptamine) is a well-established modulator of energy balance. Both pharmacological and genetic evidence implicate the serotonin 2C receptor (5-HT(2C)R) as a critical receptor mediator of serotonin's effects on ingestive behavior. Here we characterized the effect of the novel and selective 5-HT(2C)R agonist BVT.X on energy balance in obese and lean mice and report that BVT.X significantly reduces acute food intake without altering locomotor activity or oxygen consumption. In an effort to elucidate the mechanism of this effect, we examined the chemical phenotype of 5-HT(2C)R-expressing neurons in a critical brain region affecting feeding behavior, the arcuate nucleus of the hypothalamus. We show that 5-HT(2C)Rs are coexpressed with neurons containing proopiomelanocortin, known to potently affect appetite, in the arcuate nucleus of the hypothalamus of the mouse. We then demonstrate that prolonged infusion with BVT.X in obese mice significantly increases Pomc mRNA and reduces body weight, percent body fat, and initial food intake. To evaluate the functional importance of melanocortin circuitry in the effect of BVT.X on ingestive behavior, we assessed mice with disrupted melanocortin pathways. We report that mice lacking the melanocortin 4 receptor are not responsive to BVT.X-induced hypophagia, demonstrating that melanocortins acting on melanocortin 4 receptor are a requisite downstream pathway for 5-HT(2C)R agonists to exert effects on food intake. The data presented here not only indicate that the novel 5-HT(2C)R agonist BVT.X warrants further investigation as a treatment for obesity but also elucidate specific neuronal pathways potently affecting energy balance through which 5-HT(2C)R agonists regulate ingestive behavior.
Figures
Figure 1
BVT.X significantly reduces food intake. Effects of BVT.X on acute intake of HFD in DIO (n = 11, average body weight 53 g; A) and ob/ob (n = 6, average body weight 51 g; B) mice. Saline (SAL) or BVT.X (20 or 60 mg/kg) were administered ip 45 min before the onset of the dark cycle. Total food intake was measured 6 h after injection (mean ±
sem
). Significant differences are indicated as follows: **, P < 0.01; ***, P < 0.001.
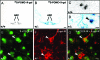
Figure 2
5-HT2CRs are coexpressed with POMC in the ARC. A, Wild-type (+/+) mice exhibit endogenous POMC mRNA using ISHH with 35S-POMC (identified by clusters of black grains) but no β-galactosidase activity (X-gal stain of blue cytoplasm) in the ARC. B, Pomc tau-lacZ+/− mice display 35S-POMC in every neuron positive for X-gal. C, Higher level magnification of wild-type+/+ mouse ARC, showing 35S-POMC grains but no X-gal stain. D, Higher-level magnification of Pomc tau-lacZ+/− mouse ARC, showing a typical cell containing both 35S-POMC grains and X-gal stain (arrow). E–F, Fluorescent IHC for 5-HT2CR (E) and β-galactosidase (F) in Pomc tau-lacZ+/− mouse ARC. G, Merge of E and F, showing cells coexpressing 5-HT2CR and β-galactosidase (arrows). Scale bar (B), 200 μm and applies to A; (D), 20 μm and applies to C; (G), 20 μm and applies to E–G.
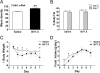
Figure 3
Prolonged 5-HT2CR agonist infusion increases POMC mRNA expression and reduces percent body fat, body weight, and initial food intake. Obese ob/ob mice (n = 8, mean body weight 46 g) were treated with saline or BVT.X (60 mg/kg·d for 7 d, sc minipump). A, Prolonged BVT.X treatment increased POMC mRNA in the ARC, compared with saline treatment, as determined by densitometry analysis after ISHH with a 35S-labeled antisense POMC riboprobe. Prolonged BVT.X treatment significantly decreased percent body fat as determined by DEXA (B) and body weight (grams) (C), compared with pretreatment levels. D, BVT.X treatment decreased chow pellet intake on d 1 and 2, but no further significant differences were observed. Data are presented as mean ±
sem
. Significant differences are indicated as follows: *, P < 0.05; **, P < 0.01.
Figure 4
BVT.X significantly decreases food intake in wild-type but not Mc4r null mice. Wild-type (A–C) and Mc4r null littermates (n = 9, average body weight 20 g) (D–F) received an acute injection of either saline or BVT.X (60 mg/kg, ip) and were assessed for 6 h in the CLAMS apparatus. BVT.X significantly reduced 6-h food intake (powdered chow) in wild-type mice (A) but not Mc4r null mice (D). BVT.X did not significantly affect average hourly locomotor activity (horizontal counts) (B and E) or VO2 (C and F) in either genotype. Data are presented as mean ±
sem
and significant differences are indicated: *, P < 0.05.
Similar articles
- TrpC5 Mediates Acute Leptin and Serotonin Effects via Pomc Neurons.
Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, Kong X, Yu KJ, Wang RT, Chen H, Guo H, Yan J, Cunningham KA, Chang Y, Liu T, Williams KW. Gao Y, et al. Cell Rep. 2017 Jan 17;18(3):583-592. doi: 10.1016/j.celrep.2016.12.072. Cell Rep. 2017. PMID: 28099839 Free PMC article. - 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis.
Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. Xu Y, et al. Neuron. 2008 Nov 26;60(4):582-9. doi: 10.1016/j.neuron.2008.09.033. Neuron. 2008. PMID: 19038216 Free PMC article. - Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake.
D'Agostino G, Lyons D, Cristiano C, Lettieri M, Olarte-Sanchez C, Burke LK, Greenwald-Yarnell M, Cansell C, Doslikova B, Georgescu T, Martinez de Morentin PB, Myers MG Jr, Rochford JJ, Heisler LK. D'Agostino G, et al. Cell Metab. 2018 Oct 2;28(4):619-630.e5. doi: 10.1016/j.cmet.2018.07.017. Epub 2018 Aug 23. Cell Metab. 2018. PMID: 30146485 Free PMC article. - Central serotonin and melanocortin pathways regulating energy homeostasis.
Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro J, Zigman JM, Cone RD, Elmquist JK. Heisler LK, et al. Ann N Y Acad Sci. 2003 Jun;994:169-74. doi: 10.1111/j.1749-6632.2003.tb03177.x. Ann N Y Acad Sci. 2003. PMID: 12851313 Review. - 5-HT(2C) receptor agonists and the control of appetite.
Halford JC, Harrold JA. Halford JC, et al. Handb Exp Pharmacol. 2012;(209):349-56. doi: 10.1007/978-3-642-24716-3_16. Handb Exp Pharmacol. 2012. PMID: 22249823 Review.
Cited by
- Weight Loss After RYGB Is Independent of and Complementary to Serotonin 2C Receptor Signaling in Male Mice.
Carmody JS, Ahmad NN, Machineni S, Lajoie S, Kaplan LM. Carmody JS, et al. Endocrinology. 2015 Sep;156(9):3183-91. doi: 10.1210/en.2015-1226. Epub 2015 Jun 11. Endocrinology. 2015. PMID: 26066076 Free PMC article. - Pharmacogenetic Correlates of Antipsychotic-Induced Weight Gain in the Chinese Population.
Luo C, Liu J, Wang X, Mao X, Zhou H, Liu Z. Luo C, et al. Neurosci Bull. 2019 Jun;35(3):561-580. doi: 10.1007/s12264-018-0323-6. Epub 2019 Jan 3. Neurosci Bull. 2019. PMID: 30607769 Free PMC article. Review. - Unraveling the periprandial changes in brain serotonergic activity and its correlation with food intake-related neuropeptides in rainbow trout Oncorhynchus mykiss.
Chivite M, Ceinos RM, Cerdá-Reverter JM, Soengas JL, Aldegunde M, López-Patiño MA, Míguez JM. Chivite M, et al. Front Endocrinol (Lausanne). 2023 Aug 24;14:1241019. doi: 10.3389/fendo.2023.1241019. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37693350 Free PMC article. - Effect of lorcaserin on weight reduction in persons with obstructive sleep apnea (OSA): a combined subgroup analysis from three randomized, controlled clinical trials.
Fujioka K, Perdomo C, Malhotra M. Fujioka K, et al. Obes Sci Pract. 2019 Apr 24;5(3):238-245. doi: 10.1002/osp4.340. eCollection 2019 Jun. Obes Sci Pract. 2019. PMID: 31275597 Free PMC article. - Fluoxetine Modulates the Activity of Hypothalamic POMC Neurons via mTOR Signaling.
Barone I, Melani R, Mainardi M, Scabia G, Scali M, Dattilo A, Ceccarini G, Vitti P, Santini F, Maffei L, Pizzorusso T, Maffei M. Barone I, et al. Mol Neurobiol. 2018 Dec;55(12):9267-9279. doi: 10.1007/s12035-018-1052-6. Epub 2018 Apr 16. Mol Neurobiol. 2018. PMID: 29663284
References
- Breisch ST, Zemlan FP, Hoebel BG 1976 Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science 192:382–385 - PubMed
- Saller CF, Stricker EM 1976 Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science 192:385–387 - PubMed
- Guy-Grand B 1995 Clinical studies with dexfenfluramine: from past to future. Obesity Res 3(Suppl 4):491S–496S - PubMed
- Levine LR, Rosenblatt S, Bosomworth J 1987 Use of a serotonin re-uptake inhibitor, fluoxetine, in the treatment of obesity. Int J Obes 11(Suppl 3):185–190 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MRC_/Medical Research Council/United Kingdom
- DK065171/DK/NIDDK NIH HHS/United States
- 081713/WT_/Wellcome Trust/United Kingdom
- BHF_/British Heart Foundation/United Kingdom
- R01 DK065171/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous