Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression - PubMed (original) (raw)
Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression
Zhiyuan Yang et al. Mol Cell Biol. 2008 Feb.
Abstract
Brd4, a bromodomain protein capable of interacting with acetylated histones, is implicated in transmitting epigenetic memory through mitosis. It also functions as an associated factor and positive regulator of P-TEFb, a Cdk9-cyclin T1 heterodimer that stimulates transcriptional elongation by phosphorylating RNA polymerase II. In the present study, experiments were performed to determine whether these two functions of Brd4 are interrelated and, if so, how they may impact cell cycle progression. Our data demonstrate that while the P-TEFb level remains constant, the Brd4-P-TEFb interaction increases dramatically in cells progressing from late mitosis to early G(1). Concurrently, P-TEFb is recruited to chromosomes, beginning around mid- to late anaphase and before nuclear envelope/lamina formation and nuclear import of other general transcription factors. Importantly, the recruitment of P-TEFb depends on Brd4. Abrogation of this process through Brd4 knockdown reduces the binding of P-TEFb to and expression of key G(1) and growth-associated genes, leading to G(1) cell cycle arrest and apoptosis. Because P-TEFb is synonymous with productive elongation, its recruitment by Brd4 to chromosomes at late mitosis may indicate those genes whose active transcription status must be preserved across cell division.
Figures
FIG. 1.
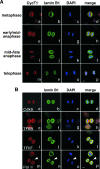
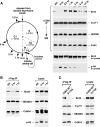
Reloading of P-TEFb onto mitotic chromosomes toward the end of mitosis. HeLa cells were cultured on coverslips, fixed, and immunostained to reveal the subcellular localizations of Cdk9 (A) and CycT1 (B) at various stages of mitosis by confocal microscopy (red). The localizations of chromosomal DNA (DAPI staining; blue) and α-tubulin (green) in the same cells are also shown. Bar, 5 μm.
FIG. 2.
Unlike most other general transcription factors and Pol II, P-TEFb is recruited to mitotic chromosomes before nuclear envelope/lamina reassembly. (A) Coimmunofluorescence followed by confocal microscopic analyses of the localizations of CycT1 (red) and lamin B1 (green) in HeLa cells at various stages of mitosis (left) was performed. Staining of chromosomal DNA by DAPI (blue) and a merge of all three stainings in the same cells are also shown. Bar, 5 μm. (B) The localizations of lamin B1, TFIIB, TFIIF (Rap30), Pol II (the cells under discussion are indicated by an arrow), and Cdk9 in HeLa cells at mid- to late anaphase were examined by coimmunofluorescence as in panel A.
FIG. 3.
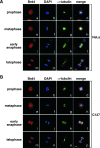
Brd4 displays cell-type-specific localization patterns during mitosis. Distributions of Brd4 in HeLa (A) and C127 cells (B) at various stages of mitosis were examined by coimmunofluorescence with antibodies against either Brd4 (red) or α-tubulin (green). Chromosomes in the same cells were stained with DAPI (blue). Cell images were obtained with a confocal microscope. Bar, 5 μm.
FIG. 4.
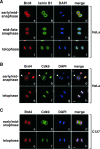
Colocalization of Brd4 and P-TEFb on mitotic chromosomes before nuclear envelope/lamina reassembly. (A) The localizations of Brd4 (red) and lamin B1 (green) in HeLa cells at various stages of mitosis (indicated on the left) were examined by coimmunofluorescence and confocal microscopy. Chromosomal DNA in the same cells was stained by DAPI (blue). Bar, 5 μm. (B and C) HeLa and C127 cells at the indicated stages of mitosis were immunostained with antibodies against Brd4 (red) and Cdk9 (green) and examined with a confocal microscope. DAPI staining of chromosomes (blue) in the same cells is also shown. Bar, 5 μm.
FIG. 5.
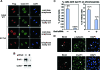
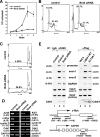
Brd4-dependent recruitment of P-TEFb to mitotic chromosomes. (A) Wild-type (WT) but not mutant Cdk9 S175A becomes associated with mitotic chromosomes before nuclear envelope/lamina reassembly. Stable HeLa-based cell lines expressing Flag-tagged wild-type Cdk9 or Cdk9 mutant S175A were immunostained at the indicated cell cycle stages (right) with antibodies against Flag (red) and lamin B1 (green). Chromosomes in the same cells were stained with DAPI (blue). Bar, 5 μm. (B) siRNA-mediated depletion of Brd4 in HeLa cells. The levels of Brd4 and Cdk9 in cells containing either an empty vector (−) or the Brd4-specific siRNA (+) were analyzed by Western blotting. (C) Reduced association of CycT1 with mitotic chromosomes in Brd4 knockdown cells. HeLa cells with (+) or without (−) the expressed Brd4 siRNA were immunostained with antibodies against CycT1 and α-tubulin to examine the behaviors of CycT1 in mitotic cells. Quantification of the percentages of cells with CycT1 on chromosomes is shown against either total cell population (left) or only mitotic cells (right). Error bar represents the mean ± standard deviation. (D) Association of CycT1 with mitotic chromosomes in cells with incomplete Brd4 depletion. Coimmunofluorescence staining was performed with anti-Brd4 (red) and anti-α-tubulin (green) or anti-CycT1 (green) antibodies in Brd4 knockdown cells. Chromosomes were stained with DAPI (blue). Arrowheads indicate cells with detectable levels of Brd4 that were in mitosis. Bar, 10 μm.
FIG. 6.
The Brd4-P-TEFb interaction significantly increases in cells progressing from late mitosis to early G1. (A) Schematic representation (left) the experimental setup. HeLa cell growth was synchronized by a double-thymidine block. Ten hours after the release from the block, mitotic cells were collected by shake-off and replated in fresh medium. Cell lysates were prepared at indicated time points and subjected to anti-Cdk9 immunoprecipitation. The lysates and the immunoprecipitates were analyzed by Western blotting (right) for the various components, as indicated to the right. (B) Mitotic cells were also collected by shake-off at 0 h from the asychronized (AS) HeLa-based cell line F1C2 expressing Flag-tagged Cdk9-f and replated in fresh medium for 1 h. The levels of the indicated components in either the cell lysate or the anti-Cdk9-f immunoprecipitates were analyzed by Western blotting. (C) The enhanced Brd4-P-TEFb interaction at late M/early G1 is disrupted by the S175A mutation in Cdk9. Stable cell lines expressing Flag-tagged wild-type Cdk9 or S175A were collected by shake-off and replated in fresh medium for 1 h. The cell lysates and anti-Flag immunoprecipitates were analyzed by Western blotting as in panel B. IP, immunoprecipitation; WT, wild type; WB, Western blotting.
FIG. 7.
Brd4 knockdown decreases the binding of P-TEFb to and expression of key early G1 genes, which leads to G1 cell cycle arrest and apoptosis. (A) HeLa cells expressing the Brd4 siRNA or containing an empty vector were placed at a low concentration (1 × 105 cells/well each) in culture medium on day 0. The numbers of cells were counted at the indicated days, with the error bars representing the mean ± standard deviation. (B) Control or Brd4 knockdown cells were stained with propidium iodide and analyzed by flow cytometry. Lin, linear. (C) Control and Brd4 knockdown cells were analyzed by flow cytometry as in panel B, with the areas corresponding to sub-G1 populations enlarged. (D) mRNAs were harvested from control or Brd4 knockdown cells and subjected to RT-PCR analyses with the primer sets specific for the indicated genes. Numbers in parentheses indicate the relative reduction in mRNA levels as a result of Brd4 knockdown. (E) Brd4 knockdown or the S175A mutation in Cdk9 reduces the binding of Cdk9 to the c-Myc gene in early G1. Control or Brd4 knockdown cells (lanes 1 to 3), HeLa (lane 4), or HeLa-based cell lines expressing Cdk9-f (lane 5) or S175A-f (lane 6) in early G1 were harvested and subjected to ChIP analysis. Three regions corresponding to the promoter, exon 1, and exon 2 of the c-Myc gene (bottom), as well as an interior region of GAPDH were amplified by PCR from the input chromatin. Cdk9 or Cdk9-f associated with the immunoprecipitated chromatin was detected by Western blotting. Numbers in parentheses indicate the relative reduction caused by Brd4 knockdown or the S175A mutation. IgG, immunoglobulin G; α, anti; IP, immunoprecipitation; WT, wild type; PI, propidium iodide.
Similar articles
- Signal-induced Brd4 release from chromatin is essential for its role transition from chromatin targeting to transcriptional regulation.
Ai N, Hu X, Ding F, Yu B, Wang H, Lu X, Zhang K, Li Y, Han A, Lin W, Liu R, Chen R. Ai N, et al. Nucleic Acids Res. 2011 Dec;39(22):9592-604. doi: 10.1093/nar/gkr698. Epub 2011 Sep 2. Nucleic Acids Res. 2011. PMID: 21890894 Free PMC article. - Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription.
Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Dey A, et al. Mol Biol Cell. 2009 Dec;20(23):4899-909. doi: 10.1091/mbc.e09-05-0380. Epub 2009 Oct 7. Mol Biol Cell. 2009. PMID: 19812244 Free PMC article. - Abrogation of the Brd4-positive transcription elongation factor B complex by papillomavirus E2 protein contributes to viral oncogene repression.
Yan J, Li Q, Lievens S, Tavernier J, You J. Yan J, et al. J Virol. 2010 Jan;84(1):76-87. doi: 10.1128/JVI.01647-09. J Virol. 2010. PMID: 19846528 Free PMC article. - The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation.
Wu SY, Chiang CM. Wu SY, et al. J Biol Chem. 2007 May 4;282(18):13141-5. doi: 10.1074/jbc.R700001200. Epub 2007 Feb 28. J Biol Chem. 2007. PMID: 17329240 Review. - The multi-tasking P-TEFb complex.
Brès V, Yoh SM, Jones KA. Brès V, et al. Curr Opin Cell Biol. 2008 Jun;20(3):334-40. doi: 10.1016/j.ceb.2008.04.008. Epub 2008 May 29. Curr Opin Cell Biol. 2008. PMID: 18513937 Free PMC article. Review.
Cited by
- Epigenetic modulators as therapeutic targets in prostate cancer.
Graça I, Pereira-Silva E, Henrique R, Packham G, Crabb SJ, Jerónimo C. Graça I, et al. Clin Epigenetics. 2016 Sep 15;8:98. doi: 10.1186/s13148-016-0264-8. eCollection 2016. Clin Epigenetics. 2016. PMID: 27651838 Free PMC article. Review. - Epigenetic-Mediated Regulation of Gene Expression for Biological Control and Cancer: Fidelity of Mechanisms Governing the Cell Cycle.
El Dika M, Fritz AJ, Toor RH, Rodriguez PD, Foley SJ, Ullah R, Nie D, Banerjee B, Lohese D, Tracy KM, Glass KC, Frietze S, Ghule PN, Heath JL, Imbalzano AN, van Wijnen A, Gordon J, Lian JB, Stein JL, Stein GS. El Dika M, et al. Results Probl Cell Differ. 2022;70:375-396. doi: 10.1007/978-3-031-06573-6_13. Results Probl Cell Differ. 2022. PMID: 36348115 Free PMC article. - The Cornelia de Lange Syndrome-associated factor NIPBL interacts with BRD4 ET domain for transcription control of a common set of genes.
Luna-Peláez N, March-Díaz R, Ceballos-Chávez M, Guerrero-Martínez JA, Grazioli P, García-Gutiérrez P, Vaccari T, Massa V, Reyes JC, García-Domínguez M. Luna-Peláez N, et al. Cell Death Dis. 2019 Jul 18;10(8):548. doi: 10.1038/s41419-019-1792-x. Cell Death Dis. 2019. PMID: 31320616 Free PMC article. - BET inhibitor trotabresib in heavily pretreated patients with solid tumors and diffuse large B-cell lymphomas.
Moreno V, Vieito M, Sepulveda JM, Galvao V, Hernández-Guerrero T, Doger B, Saavedra O, Carlo-Stella C, Michot JM, Italiano A, Magagnoli M, Carpio C, Pinto A, Sarmiento R, Amoroso B, Aronchik I, Filvaroff E, Hanna B, Wei X, Nikolova Z, Braña I. Moreno V, et al. Nat Commun. 2023 Mar 13;14(1):1359. doi: 10.1038/s41467-023-36976-1. Nat Commun. 2023. PMID: 36914652 Free PMC article. Clinical Trial. - Brd4 and HEXIM1: multiple roles in P-TEFb regulation and cancer.
Chen R, Yik JH, Lew QJ, Chao SH. Chen R, et al. Biomed Res Int. 2014;2014:232870. doi: 10.1155/2014/232870. Epub 2014 Jan 29. Biomed Res Int. 2014. PMID: 24592384 Free PMC article. Review.
References
- Bonifacino, J. S., J. B. Dasso, J. Harford, K. M. Lippincott-schwartz, Yamada, and K. S. Morgan (ed.). 1998. Current protocols in cell biology. John Wiley and Sons, New York, NY.
- Goldman, R. D., Y. Gruenbaum, R. D. Moir, D. K. Shumaker, and T. P. Spann. 2002. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16533-547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous