MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover - PubMed (original) (raw)
MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover
Linda Wordeman et al. J Cell Biol. 2007.
Abstract
Mitotic centromere-associated kinesin (MCAK)/Kif2C is the most potent microtubule (MT)-destabilizing enzyme identified thus far. However, MCAK's function at the centromere has remained mechanistically elusive because of interference from cytoplasmic MCAK's global regulation of MT dynamics. In this study, we present MCAK chimeras and mutants designed to target centromere-associated MCAK for mechanistic analysis. Live imaging reveals that depletion of centromere-associated MCAK considerably decreases the directional coordination between sister kinetochores. Sister centromere directional antagonism results in decreased movement speed and increased tension. Sister centromeres appear unable to detach from kinetochore MTs efficiently in response to directional switching cues during oscillatory movement. These effects are reversed by anchoring ectopic MCAK to the centromere. We propose that MCAK increases the turnover of kinetochore MTs at all centromeres to coordinate directional switching between sister centromeres and facilitate smooth translocation. This may contribute to error correction during chromosome segregation either directly via slow MT turnover or indirectly by mechanical release of MTs during facilitated movement.
Figures
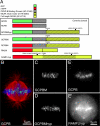
Figure 1.
Diagram of constructs and localization in mitotic cells. (A) EGFP (green) was fused to the N terminus of the minimal centromere-binding domain of CENP-B (gray) to mark centromeres (GCPB). This construct was fused to the minimal neck plus motor domain of MCAK (yellow/black crosshatch), which possessed an S186A mutation preventing phosphorylation by aurora B kinase (GCPBM). The control for this construct possesses three point mutations inactivating the motor domain (GCPBMhyp). Finally, full-length MCAK (yellow) with all aurora B phosphorylation sites mutated to alanine and the motor domain inactivated was linked to mRFP for use as a dominant-negative construct for endogenous active MCAK (RAMFLhyp). (B) GCPB localizes specifically to centromeres in HeLa cells colabeled with antitubulin (red) and Hoechst (blue). (C–E) Z projections of live metaphase HeLa cells expressing the constructs GCPBM (C) and GCPBMhyp (D), which localize specifically to centromeres in live HeLa cells. (E and E′) A live HeLa cell cotransfected with GCPB (E) and RAMFLhyp (E′). Centromere behavior is scored by observing the GCPB channel in dual-transfected cells.
Figure 2.
MCAK suppresses tension in a dose-dependent manner. (A) Addition of exogenous MCAK (GCPBM) to centromeres decreases centromere separation significantly from controls (GCPB and GCPBMhyp) in living cells (P < 0.0001). Expression of mRFP motorless MCAK (RMLM), RAMFLhyp, or depletion of MCAK with siRNA significantly increases sister centromere separation (as visualized by GCPB; P < 0.0001). Between five and eight live sister centromeres per cell were measured for 5–15 successive frames (20 s per frame). Each bar (SEM [error bars] is shown) represents three to six different cells from at least two different experiments on different days. (B) Sister centromeres in metaphase cells expressing GCPBM appear quite close together. See Video 1 (available at
http://www.jcb.org/cgi/content/full/jcb.200707120/DC1
). (C) Sister centromeres in metaphase cells coexpressing GCPB and RAMFLhyp appear to be under significantly more tension (P < 0.0001). See Video 2. (D) Histograms of sister centromere separation for GCPB, GCPBM, GCPBMhyp, and RAMFLhyp. Overlaid comparisons are shown between GCPBM and GCPBMhyp, the two controls (which are not significantly different; P = 0.40), and GCPB- and GCPB/RAMFLhyp-coexpressing cells.
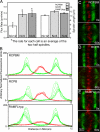
Figure 3.
MCAK enrichment on centromeres antagonizes MT flux. (A) Addition of MCAK to centromeres significantly decreases the flux rate of kinetochore fibers (left axis; P = 0.0003). Depletion of centromeric MCAK does not affect the rate of flux (P = 0.85). Overall spindle length is also slightly decreased in MCAK-enriched centromeres (right axis). MCAK depletion did not significantly alter spindle length (P = 0.18). Error bars represent SEM. (B) Successive 10-s line scans of photoactivated tubulin (green) in control (RCPB) and MCAK-enriched (RCPBM) cells. Flux is inhibited in cells possessing MCAK-enriched centromeres. (C) A representative RCPBM cell at 0 s (right after photoactivation) and 60 s later. (D) A representative RCPB cell at t = 0 and t = 60 s. (E) A representative RAMFLhyp cell at t = 0 and t = 60 s.
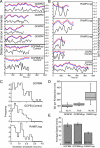
Figure 4.
Tracks of sister centromere oscillations over time. (A) Representative traces of a pair of sister centromeres (red and blue) tracked over time. Each tick (left axis) corresponds to a distance of 2 μm. The right axis shows sister centromere separation plotted below (black line). GCPBM centromeres exhibit more directional persistence and greater oscillation amplitude than control (GCBMhyp) centromeres. (B) Similar traces of sister centromeres (red and blue) tracked from cells expressing RAMFLhyp versus GCPB controls. MCAK-depleted centromeres (RAMFLhyp) are not as well coordinated in directionality. (C) Histograms of the oscillation amplitudes of pooled data from three to six centromeres each in three to six different cells filmed and transfected on different days. The oscillation amplitude of GCPBM is significantly greater than GCPB (control) at the 95% confidence interval (P = 0.033). The mean oscillation amplitude of RAMFLMhyp is not substantially different from controls because the distribution is bimodal. (D) Box plots of the SD of the sister centromere separation of individual sister centromere pairs over 8–15 frames. N, number of kinetochores. (E) Mean distance over time of oscillating metaphase centromeres in control (GCPBMhyp), MCAK-enriched (GCPBM), or MCAK-depleted (RAMFLhyp) cells. Error bars represent SEM.
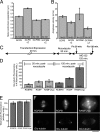
Figure 5.
MCAK opposes attachment at the kinetochore. (A) Additional MCAK on the centromere rescues the increase in tension of bioriented kinetochores caused by transient aurora B kinase inactivation. N, number of individual live measurements resulting from six bioriented kinetochores in each of three metaphase cells. (B) Additional MCAK on the centromere does not rescue the decrease in anaphase chromosome segregation velocity caused by aurora B kinase inhibition. This suggests that the increase in tension is not solely caused by increased numbers of lateral merotelic MT–kinetochore interactions. N, number of individual live measurements resulting from five kinetochores in each of three to five cells. (C) Method for retyrosinating CHO tubulin to baseline before spindle assembly. Release of cells from nocodazole permits MT assembly and detyrosination (increase in glu-tubulin). (D) Cells exhibit significantly more kinetochore fiber detyrosinated tubulin (glu-tubulin) when depleted of MCAK (P < 0.0001). N, number of cells. (E) Total tubulin fluorescence measured from the same region that the glu-tubulin fluorescence was measured (D, dark bars). (F) Glu-tubulin labeling of representative cells transfected with RCBPM, RCPB, or RAMFLhyp after 120-min nocodazole reversal. Error bars represent SEM. Bar, 0.5 μm.
Figure 6.
MCAK facilitates error correction. (A) Addition of RCPBM to the centromere significantly reduced the percentage of lagging chromosomes in anaphase CHO cells, whereas expression of dominant-negative RFLMhyp significantly increased the percentage of lagging chromosomes (P = 0.02). Error bars represent SEM. (B) Model for the role of MCAK in regulating MT attachment in translocating kinetochores. MT-dependent chromosome movement must, by necessity, balance the breakage of binding sites and the reformation of new sites to maintain attachment. MCAK may use its depolymerizing activity to release a small proportion of MTs from the kinetochore. This facilitates and synchronizes movement by modulating the number of attachment sites between the kinetochore and the MTs. We believe that MCAK provides this activity to both the leading and trailing kinetochore. Improved centromere synchronization, increased MT turnover, or both activities together may facilitate error correction.
Similar articles
- Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity.
Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Lan W, et al. Curr Biol. 2004 Feb 17;14(4):273-86. doi: 10.1016/j.cub.2004.01.055. Curr Biol. 2004. PMID: 14972678 - Aurora B regulates MCAK at the mitotic centromere.
Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Andrews PD, et al. Dev Cell. 2004 Feb;6(2):253-68. doi: 10.1016/s1534-5807(04)00025-5. Dev Cell. 2004. PMID: 14960279 - A perikinetochoric ring defined by MCAK and Aurora-B as a novel centromere domain.
Parra MT, Gómez R, Viera A, Page J, Calvente A, Wordeman L, Rufas JS, Suja JA. Parra MT, et al. PLoS Genet. 2006 Jun 2;2(6):e84. doi: 10.1371/journal.pgen.0020084. Epub 2006 Apr 20. PLoS Genet. 2006. PMID: 16741559 Free PMC article. - Mitotic centromere-associated kinesin (MCAK): a potential cancer drug target.
Sanhaji M, Friel CT, Wordeman L, Louwen F, Yuan J. Sanhaji M, et al. Oncotarget. 2011 Dec;2(12):935-47. doi: 10.18632/oncotarget.416. Oncotarget. 2011. PMID: 22249213 Free PMC article. Review. - Molecular insight into the regulation and function of MCAK.
Ritter A, Kreis NN, Louwen F, Wordeman L, Yuan J. Ritter A, et al. Crit Rev Biochem Mol Biol. 2015 Jul-Aug;51(4):228-45. doi: 10.1080/10409238.2016.1178705. Epub 2016 May 5. Crit Rev Biochem Mol Biol. 2015. PMID: 27146484 Review.
Cited by
- Probing microtubule polymerisation state at single kinetochores during metaphase chromosome motion.
Armond JW, Vladimirou E, Erent M, McAinsh AD, Burroughs NJ. Armond JW, et al. J Cell Sci. 2015 May 15;128(10):1991-2001. doi: 10.1242/jcs.168682. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908867 Free PMC article. - Springs, clutches and motors: driving forward kinetochore mechanism by modelling.
Vladimirou E, Harry E, Burroughs N, McAinsh AD. Vladimirou E, et al. Chromosome Res. 2011 Apr;19(3):409-21. doi: 10.1007/s10577-011-9191-x. Chromosome Res. 2011. PMID: 21331796 Free PMC article. Review. - Mechanical coupling coordinates microtubule growth.
Leeds BK, Kostello KF, Liu YY, Nelson CR, Biggins S, Asbury CL. Leeds BK, et al. bioRxiv [Preprint]. 2023 Oct 17:2023.06.29.547092. doi: 10.1101/2023.06.29.547092. bioRxiv. 2023. PMID: 37905093 Free PMC article. Updated. Preprint. - Mass spec studio for integrative structural biology.
Rey M, Sarpe V, Burns KM, Buse J, Baker CA, van Dijk M, Wordeman L, Bonvin AM, Schriemer DC. Rey M, et al. Structure. 2014 Oct 7;22(10):1538-48. doi: 10.1016/j.str.2014.08.013. Epub 2014 Sep 18. Structure. 2014. PMID: 25242457 Free PMC article. - MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics.
Rizk RS, Bohannon KP, Wetzel LA, Powers J, Shaw SL, Walczak CE. Rizk RS, et al. Mol Biol Cell. 2009 Mar;20(6):1639-51. doi: 10.1091/mbc.e08-09-0985. Epub 2009 Jan 21. Mol Biol Cell. 2009. PMID: 19158381 Free PMC article.
References
- Andrews, P.D., Y. Ovechkina, N. Morrice, M. Wagenbach, K. Duncan, L. Wordeman, and J.R. Swedlow. 2004. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 6:253–268. - PubMed
- Cimini, D., B. Moree, J.C. Canman, and E.D. Salmon. 2003. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116:4213–4225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials