Fat-1 transgenic mice: a new model for omega-3 research - PubMed (original) (raw)
Fat-1 transgenic mice: a new model for omega-3 research
Jing X Kang. Prostaglandins Leukot Essent Fatty Acids. 2007 Nov-Dec.
Abstract
An appropriate animal model that can eliminate confounding factors of diet would be very helpful for evaluation of the health effects of nutrients such as n-3 fatty acids. We recently generated a fat-1 transgenic mouse expressing the Caenorhabditis elegans fat-1 gene encoding an n-3 fatty acid desaturase that converts n-6 to n-3 fatty acids (which is absent in mammals). The fat-1 transgenic mice are capable of producing n-3 fatty acids from the n-6 type, leading to abundant n-3 fatty acids with reduced levels of n-6 fatty acids in their organs and tissues, without the need of a dietary n-3 supply. Feeding an identical diet (high in n-6) to the transgenic and wild-type littermates can produce different fatty acid profiles in these animals. Thus, this model allows well-controlled studies to be performed, without the interference of the potential confounding factors of diet. The transgenic mice are now being used widely and are emerging as a new tool for studying the benefits of n-3 fatty acids and the molecular mechanisms of their action.
Figures
Fig. 1
Conversion of n-6 fatty acids (FA) to n-3 fatty acids by an n-3 desaturase that does not exist in mammalian cells. The n-3 desaturase can catalyze introduction of a double bond into n-6 fatty acids at the n-3 position of their hydrocarbon chains to form n-3 fatty acids.
Fig. 2
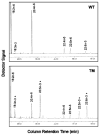
Partial gas chromatograph traces showing the polyunsaturated fatty acid profiles of total lipids extracted from skeletal muscles of a wild-type mouse (WT, upper panel) and a fat-1 transgenic mouse (TM, lower panel). Both the wild-type and transgenic mice were 8-week-old females and fed on the same diet, which is high in n-6 but low in n-3 fatty acids. Note, the levels of n-6 fatty acids (18:2n-6, 20:4n-6, 22:4n-6 and 22:5n-6) are remarkably lower whereas n-3 fatty acids (marked with *) are abundant in the transgenic muscle (lower panel) compared with the wild type muscle in which there is very little n-3 fatty acids (upper panel).
Similar articles
- Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids.
Kang JX, Wang J, Wu L, Kang ZB. Kang JX, et al. Nature. 2004 Feb 5;427(6974):504. doi: 10.1038/427504a. Nature. 2004. PMID: 14765186 - Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids.
Xia S, Lu Y, Wang J, He C, Hong S, Serhan CN, Kang JX. Xia S, et al. Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12499-504. doi: 10.1073/pnas.0605394103. Epub 2006 Aug 3. Proc Natl Acad Sci U S A. 2006. PMID: 16888035 Free PMC article. - N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis.
Kim JY, Lim K, Kim KH, Kim JH, Choi JS, Shim SC. Kim JY, et al. PLoS One. 2018 Mar 15;13(3):e0194331. doi: 10.1371/journal.pone.0194331. eCollection 2018. PLoS One. 2018. PMID: 29543869 Free PMC article. - n-3 polyunsaturated fatty acids and HER2-positive breast cancer: interest of the fat-1 transgenic mouse model over conventional dietary supplementation.
Zou Z, Bidu C, Bellenger S, Narce M, Bellenger J. Zou Z, et al. Biochimie. 2014 Jan;96:22-7. doi: 10.1016/j.biochi.2013.08.021. Epub 2013 Sep 6. Biochimie. 2014. PMID: 24012777 Review. - From fat to fat-1: a tale of omega-3 fatty acids.
Kang JX. Kang JX. J Membr Biol. 2005 Jul;206(2):165-72. doi: 10.1007/s00232-005-0790-3. J Membr Biol. 2005. PMID: 16456726 Review. No abstract available.
Cited by
- The effects of omega-3 polyunsaturated Fatty Acid consumption on mammary carcinogenesis.
Witte TR, Hardman WE. Witte TR, et al. Lipids. 2015 May;50(5):437-46. doi: 10.1007/s11745-015-4011-2. Epub 2015 Apr 10. Lipids. 2015. PMID: 25860692 Review. - Inhibition of Pancreatic Carcinoma Growth Through Enhancing ω-3 Epoxy Polyunsaturated Fatty Acid Profile by Inhibition of Soluble Epoxide Hydrolase.
Xia R, Sun L, Liao J, Li H, You X, Xu D, Yang J, Hwang SH, Jones RD, Hammock B, Yang GY. Xia R, et al. Anticancer Res. 2019 Jul;39(7):3651-3660. doi: 10.21873/anticanres.13513. Anticancer Res. 2019. PMID: 31262891 Free PMC article. - Lipid and Lipoprotein Metabolism in Microglia.
Loving BA, Bruce KD. Loving BA, et al. Front Physiol. 2020 Apr 28;11:393. doi: 10.3389/fphys.2020.00393. eCollection 2020. Front Physiol. 2020. PMID: 32411016 Free PMC article. Review. - Peroxisome Proliferator-Activated Receptor Expression in Murine Models and Humans with Age-related Macular Degeneration.
Herzlich AA, Ding X, Shen D, Ross RJ, Tuo J, Chan CC. Herzlich AA, et al. Open Biol J. 2009 Jan 1;2:141-148. doi: 10.2174/1874196700902010141. Open Biol J. 2009. PMID: 21152244 Free PMC article. - Effect of dietary docosahexaenoic acid on rhodopsin content and packing in photoreceptor cell membranes.
Senapati S, Gragg M, Samuels IS, Parmar VM, Maeda A, Park PS. Senapati S, et al. Biochim Biophys Acta Biomembr. 2018 Jun;1860(6):1403-1413. doi: 10.1016/j.bbamem.2018.03.030. Epub 2018 Apr 4. Biochim Biophys Acta Biomembr. 2018. PMID: 29626443 Free PMC article.
References
- Balk EM, Horsley TA, Newberry SJ, Lichtenstein AH, Yetley EA, Schachter HM, Moher D, MacLean CH, Lau J. A collaborative effort to apply the evidence-based review process to the field of nutrition: challenges, benefits, and lessons learned. Am J Clin Nutr. 2007;85(6):1448–1456. - PubMed
- Kang JX, Wang J, Wu L, Kang ZB. Fat-1 transgenic mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases