Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway - PubMed (original) (raw)
Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway
Eric T Sawey et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Acinar-to-ductal metaplasia in the pancreas is associated with an increased risk for tumorigenesis. Molecular dissection of this process in vitro has shown that primary acinar cells, in response to EGF receptor ligands, can transdifferentiate into duct-like epithelia, passing through a nestin-positive intermediate, in a Notch pathway-dependent manner. Here, we show that in vitro acinar transdifferentiation depends on matrix metalloproteinase 7 (MMP-7), a proteinase expressed in most metaplastic epithelia in vivo. MMP-7 was found to be required for Notch activation, which leads to dedifferentiation of acinar cells to the nestin-positive transitional cell. Besides being necessary for acinar transdifferentiation, it was found that MMP-7 activity was sufficient to induce the process, indicating that molecular signals capable of initiating MMP-7 expression also have the potential to induce formation of metaplastic epithelia in the pancreas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
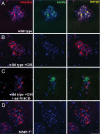
MMP-7 is expressed in primary acinar cells treated with TGF-α. Pancreatic explants from wild-type mice embedded in collagen were treated with TGF-α. MMP-7 expression (green) was confirmed by immunofluorescence 1 (A), 2 (B), and 3 days (C) after culture, respectively. Staining was evident in the majority of acini on day 2. (Scale bar, 50 μm.) Cells were costained for amylase (red) and DAPI (blue). Data are representative of five independent experiments.
Fig. 2.
MMP-7 is required for acinar-to-ductal transdifferentiation. Primary acinar cells from wild-type (A–C) and MMP-7−/− (D–I) mice were embedded in collagen and treated with TGF-α. Coimmunofluorescence for the acinar cell marker amylase (red) and the duct cell marker cytokeratin-19 (green) was performed on cells fixed on day 0 (A, D, and G), day 3 (B, E, and H), and day 5 (C, F, and I). TGF-α treatment of wild-type cultures induced acinar-to-ductal transdifferentiation by day 5 (C). TGF-α treatment of MMP-7−/− acinar cells showed virtually no conversion to ductal structures (D–F). Addition of 200 ng/ml rMMP-7 with TGF-α to MMP-7−/− acinar cells (G–I) restored transdifferentiation. (Scale bar, 100 μm.) DAPI is shown in blue. Data are representative of >10 independent experiments.
Fig. 3.
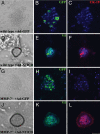
Notch and MMP-7 are required for transition to the nestin-positive intermediate. Nestin immunofluorescence (green) was detectable on day 3 in wild-type cultures treated with DMSO (A), but not in cultures treated with 20 μM the γ-secretase inhibitor, WPE III-31C (GSI) (B). Infection with an adenovirus encoding a constitutively active V5-tagged Notch-1 intracellular domain (Ad-N1ICD-V5) bypassed the GSI, allowing nestin expression (C). Day 3 MMP-7−/− cultures did not show nestin immunoreactivity (D). (Scale bar, 50 μm.) Shown are amylase in red and DAPI in blue. Data are representative of at least three independent experiments.
Fig. 4.
Constitutively active Notch bypasses the requirement for MMP-7 in acinar transdifferentiation. Primary acinar cells from wild-type (A–F) or MMP-7−/− mice (G–L) were infected either with Ad-GFP, an adenovirus encoding GFP (A–C and G–I) or Ad-N1ICD-V5 (D–F and J–L) and embedded in collagen. After 3 days in culture, transdifferentiation in N1ICD cultures was evident (D and J) and was coincident with successful infection, confirmed by V5 immunofluorescence (E and K). Transdifferentiation was confirmed by immunofluorescence for CK-19 (F and L). (Scale bar, 50 μm.) DAPI is shown in blue. Data are representative of three independent experiments.
Fig. 5.
MMP-7 activity is sufficient to induce Notch-dependent acinar-to-ductal transdifferentiation. (A and B) Primary acinar cells from wild-type mice were treated with 200 ng/ml rMMP-7 along with DMSO (A) or 20 μM WPE III-31C (GSI) (B). Coimmunofluorescence for amylase (red) and CK-19 (green) was performed on day 5. (C and D) Primary acinar cells infected with an adenovirus encoding a dominant-negative RBP-Jκ also blocked transdifferentiation induced by coinfection with Ad-N1ICD-V5 (amylase in red, V5 in green) (C) or by rMMP-7 (amylase in red, CK-19 in green) (D). (Scale bar, 50 μm.) DAPI is shown in blue. Data are representative of at least three independent experiments.
Fig. 6.
MMP-7 activity induces γ-secretase cleavage of Notch-1. (A–C) Immunoblots for the γ-secretase-cleaved form of Notch-1 (Cleaved N1 Val-1744). (A) COS-7 cells expressing Notch-1 were treated with 100 ng/ml pro-MMP-7, 50 ng/ml active rMMP-7 with DMSO, or 20 μM WPE III-31C. (B) COS-7 cells expressing Notch-1 were treated with 200 ng/ml rMMP-7 for 0, 2, 4, 6, and 18 h. (C) COS-7 cells expressing Notch-1 were treated with varied rMMP-7 concentrations for 4 h. (D) COS-7 cells expressing Notch-1 with C-terminal V5 were treated with 200 ng/ml for 18 h. Data are representative of five independent experiments.
Fig. 7.
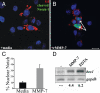
MMP-7 activity leads to nuclear translocation of N1ICD and expression of hes-1. (A and B) COS-7 cells expressing full-length Notch-1 with a C-terminal V5 tag were treated for 4 h with MMP-7. Shown is immunofluorescence for Cleaved N1 Val-1744 antibody (green) and V5 antibody (red). (Scale bar, 20 μm.) Cells were treated for 4 h with medium alone (A) or with rMMP-7 (B). Arrows indicate cells with nuclear cleaved Notch-1. DAPI is shown in blue. (C) Quantification of Notch-1 nuclear translocation. Percentage represents cells with nuclear Notch-1 divided by total cells expressing Notch-1. (D) RT-PCR for hes1 from COS-7 cells expressing Notch-1 treated with MMP-7 or EDTA. Numbers represent fold expression relative to medium alone. Data are representative of five independent experiments.
Fig. 8.
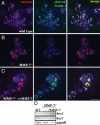
MMP-7 is required for Notch activation in acinar explants. (A–C) Acinar explants immunostained for Cleaved N1 Val-1744 (green) and amylase (red) on day 3. Wild-type acinar cells exhibited Notch cleavage (A), whereas MMP-7−/− cells did not (B). Inclusion of 200 ng/ml rMMP-7 rescued Notch cleavage in MMP-7−/− acinar cells (C). (Scale bar, 50 μm.) DAPI is shown in blue. (D) RT-PCR for hes1 and hey1 showed increased Notch activation in wild-type acinar cells and MMP-7−/− cells treated with rMMP-7, relative to MMP-7−/− cells. Data are representative of five independent experiments.
Similar articles
- NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas.
Chen NM, Singh G, Koenig A, Liou GY, Storz P, Zhang JS, Regul L, Nagarajan S, Kühnemuth B, Johnsen SA, Hebrok M, Siveke J, Billadeau DD, Ellenrieder V, Hessmann E. Chen NM, et al. Gastroenterology. 2015 May;148(5):1024-1034.e9. doi: 10.1053/j.gastro.2015.01.033. Epub 2015 Jan 23. Gastroenterology. 2015. PMID: 25623042 Free PMC article. - Metalloproteinases and cell fate: Notch just ADAMs anymore.
Sawey ET, Crawford HC. Sawey ET, et al. Cell Cycle. 2008 Mar 1;7(5):566-9. doi: 10.4161/cc.7.5.5531. Epub 2007 Dec 29. Cell Cycle. 2008. PMID: 18239463 - Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas.
Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Crawford HC, et al. J Clin Invest. 2002 Jun;109(11):1437-44. doi: 10.1172/JCI15051. J Clin Invest. 2002. PMID: 12045257 Free PMC article. - Transition to pancreatic cancer in response to carcinogen.
Bockman DE. Bockman DE. Langenbecks Arch Surg. 2008 Jul;393(4):557-60. doi: 10.1007/s00423-007-0274-2. Epub 2008 Jan 12. Langenbecks Arch Surg. 2008. PMID: 18189145 Review. - Epithelial differentiation in pancreatic development and neoplasia: new niches for nestin and Notch.
Leach SD. Leach SD. J Clin Gastroenterol. 2005 Apr;39(4 Suppl 2):S78-82. doi: 10.1097/01.mcg.0000155547.83901.a3. J Clin Gastroenterol. 2005. PMID: 15758664 Review.
Cited by
- Acinar cell carcinoma: a report of 19 cases with a brief review of the literature.
Wang Y, Wang S, Zhou X, Zhou H, Cui Y, Li Q, Zhang L. Wang Y, et al. World J Surg Oncol. 2016 Jun 28;14(1):172. doi: 10.1186/s12957-016-0919-0. World J Surg Oncol. 2016. PMID: 27352960 Free PMC article. Review. - Remodeling the exocrine pancreas at metamorphosis in Xenopus laevis.
Mukhi S, Mao J, Brown DD. Mukhi S, et al. Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):8962-7. doi: 10.1073/pnas.0803569105. Epub 2008 Jun 23. Proc Natl Acad Sci U S A. 2008. PMID: 18574144 Free PMC article. - GRP78 haploinsufficiency suppresses acinar-to-ductal metaplasia, signaling, and mutant _Kras_-driven pancreatic tumorigenesis in mice.
Shen J, Ha DP, Zhu G, Rangel DF, Kobielak A, Gill PS, Groshen S, Dubeau L, Lee AS. Shen J, et al. Proc Natl Acad Sci U S A. 2017 May 16;114(20):E4020-E4029. doi: 10.1073/pnas.1616060114. Epub 2017 May 1. Proc Natl Acad Sci U S A. 2017. PMID: 28461470 Free PMC article. - Cytokine CCL9 Mediates Oncogenic KRAS-Induced Pancreatic Acinar-to-Ductal Metaplasia by Promoting Reactive Oxygen Species and Metalloproteinases.
Liou GY, Byrd CJ, Storz P, Messex JK. Liou GY, et al. Int J Mol Sci. 2024 Apr 26;25(9):4726. doi: 10.3390/ijms25094726. Int J Mol Sci. 2024. PMID: 38731942 Free PMC article. - A Novel PAK1-Notch1 Axis Regulates Crypt Homeostasis in Intestinal Inflammation.
Frick A, Khare V, Jimenez K, Dammann K, Lang M, Krnjic A, Gmainer C, Baumgartner M, Mesteri I, Gasche C. Frick A, et al. Cell Mol Gastroenterol Hepatol. 2021;11(3):892-907.e1. doi: 10.1016/j.jcmgh.2020.11.001. Epub 2020 Nov 12. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33189893 Free PMC article.
References
- Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz R. Mod Pathol. 2002;15:441–447. - PubMed
- Lowenfels AB, Maisonneuve P. Best Pract Res Clin Gastroenterol. 2006;20:197–209. - PubMed
- Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ, Jr, Wright CV, et al. Gastroenterology. 1999;117:1416–1426. - PubMed
- Chambers AF, Matrisian LM. J Natl Cancer Inst. 1997;89:1260–1270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical