Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons - PubMed (original) (raw)
Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons
Tuan V Bui et al. J Neurophysiol. 2008 Feb.
Abstract
In some motoneurons, L-type Ca2+ channels that partly mediate persistent inward currents (PICs) have been estimated to be arranged in 50- to 200-microm-long discrete regions in the dendrites, centered 100 to 400 microm from the soma. As a consequence of this nonuniform distribution, the interaction between synaptic inputs to motoneurons and these channels may vary according to the distribution of the synapses. For instance, >93% of synapses from Renshaw cells have been observed to be located 65 to 470 microm away from the cell body of motoneurons. Our goal was to assess whether Renshaw cell synapses are distributed in a position to more effectively control the activation of the L-type Ca2+ channels. Using compartmental models of motoneurons with L-type Ca2+ channels distributed in 100-microm-long hot spots centered 100 to 400 microm away from the soma, we compared the inhibition generated by four distributions of inhibitory synapses: proximal, distal, uniform, and one based on the location of Renshaw cell synapses on motoneurons. Regardless of whether the synapses were activated tonically or transiently, in the presence of L-type Ca2+ channels, inhibitory synapses distributed according to the Renshaw cell synapse distribution generate the largest inhibitory currents. The effectiveness of a particular distribution of inhibitory synapses in the presence of PICs depends on their ability to deactivate the channels underlying PICs, which is influenced not only by the superposition between synapses and channels, but also by the distance away from the somatic voltage clamp.
Figures
FIG. 1
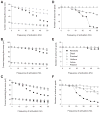
Current reaching the cell body and number of active L-type Ca2+ channel hot spots for 3 motoneuron models with 4 distributions of inhibitory synapses activated at various frequencies and a specific membrane resistivity (_R_m) of 15,000 Ω·cm2. The cell body was clamped at −55 mV and excitatory synaptic inputs were activated at 50 Hz. Current reaching the cell body for LAD5-4 (A), LVN2-1 (B), and LVN4-1 (C). Solid lines indicate simulations with L-type Ca2+ channels distributed in hot spots. Dashed lines indicate simulations with no L-type Ca2+ channels present. D_–_F: number of active L-type Ca2+ channel hot spots for LAD5-4 (D), LVN2-1 (E), and LVN4-1 (F). A hot spot is defined as active if the open probability of its channels is >0.5. Dotted lines indicate the total number of L-type Ca2+ channel hot spots in each model. In E, the total number of L-type Ca2+ channel hot spots is 20.
FIG. 2
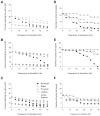
Current reaching the cell body and number of active L-type Ca2+ channel hot spots for 3 motoneuron models with 4 distributions of inhibitory synapses activated at various frequencies and an _R_m of 5,000 Ω·cm2. The cell body was clamped at −55 mV and excitatory synaptic inputs were activated at 50 Hz. Current reaching the cell body for LAD5-4 (A), LVN2-1 (B), and LVN4-1 (C). Solid lines indicate simulations with L-type Ca2+ channels distributed in hot spots. Dashed lines indicate simulations with no L-type Ca2+ channels present. D_–_F: number of active L-type Ca2+ channel hot spots for LAD5-4 (D), LVN2-1 (E), and LVN4-1 (F). A hot spot is defined as active if the open probability of its channels is >0.5. Dotted lines indicate the total number of L-type Ca2+ channel hot spots in each model.
FIG. 3
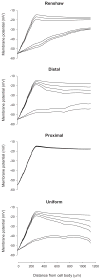
Membrane potential profile of a dendritic path from cell body to terminal end of LAD5-4 during different levels of steady-state inhibitory synaptic activity under the 4 different distributions. _R_m was set at 5,000 Ω·cm2. The cell body was clamped at −55 mV and excitatory synaptic inputs were activated at 50 Hz. For each set of membrane potential profiles corresponding to a particular distribution of inhibitory synaptic activity, the profiles from top to bottom depict the responses to steady-state inhibition at frequencies of 0, 10, 20, 30, 40, and 50 Hz, respectively.
FIG. 4
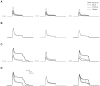
Transient inhibitory postsynaptic currents (IPSCs) generated by synapses distributed according to Renshaw, proximal, distal, and uniform synapse distribution with membrane potential clamped at −55 mV and tonically activated excitatory synapses for motoneuron models LAD5-4 (left), LVN2-1 (center), and LVN4-1 (right). _R_m was set to a value of 5,000 Ω·cm2. Currents are injected by a somatic voltage clamp; therefore downward currents are depolarizing and upward currents are hyperpolarizing. A: IPSCs in motoneurons with no L-type Ca2+ channels. The current reaching the soma in the absence of any inhibition is denoted to the left of each trace. B: IPSCs from A normalized to the peak of the largest IPSC in each model. C: IPSCs in motoneurons with L-type Ca2+ channels included in the models. The current reaching the soma in the absence of any inhibition is denoted to the left of each trace. D: IPSCs from C normalized to the peak of the largest IPSC in each motoneuron.
Similar articles
- Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents.
Bui TV, Grande G, Rose PK. Bui TV, et al. J Neurophysiol. 2008 Feb;99(2):571-82. doi: 10.1152/jn.00717.2007. Epub 2007 Nov 28. J Neurophysiol. 2008. PMID: 18046007 Free PMC article. - Computational estimation of the distribution of L-type Ca(2+) channels in motoneurons based on variable threshold of activation of persistent inward currents.
Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Bui TV, et al. J Neurophysiol. 2006 Jan;95(1):225-41. doi: 10.1152/jn.00646.2005. Epub 2005 Nov 2. J Neurophysiol. 2006. PMID: 16267115 - Estimates of the location of L-type Ca2+ channels in motoneurons of different sizes: a computational study.
Grande G, Bui TV, Rose PK. Grande G, et al. J Neurophysiol. 2007 Jun;97(6):4023-35. doi: 10.1152/jn.00044.2007. Epub 2007 Apr 11. J Neurophysiol. 2007. PMID: 17428909 Free PMC article. - Synaptic integration in motoneurons with hyper-excitable dendrites.
Heckman CJ, Kuo JJ, Johnson MD. Heckman CJ, et al. Can J Physiol Pharmacol. 2004 Aug-Sep;82(8-9):549-55. doi: 10.1139/y04-046. Can J Physiol Pharmacol. 2004. PMID: 15523512 Review. - How voltage-gated ion channels alter the functional properties of ganglion and amacrine cell dendrites.
Miller RF, Stenback K, Henderson D, Sikora M. Miller RF, et al. Arch Ital Biol. 2002 Oct;140(4):347-59. Arch Ital Biol. 2002. PMID: 12228988 Review.
Cited by
- Modulation of inhibitory strength and kinetics facilitates regulation of persistent inward currents and motoneuron excitability following spinal cord injury.
Venugopal S, Hamm TM, Crook SM, Jung R. Venugopal S, et al. J Neurophysiol. 2011 Nov;106(5):2167-79. doi: 10.1152/jn.00359.2011. Epub 2011 Jul 20. J Neurophysiol. 2011. PMID: 21775715 Free PMC article. - Electrical Properties of Adult Mammalian Motoneurons.
Smith CC, Brownstone RM. Smith CC, et al. Adv Neurobiol. 2022;28:191-232. doi: 10.1007/978-3-031-07167-6_9. Adv Neurobiol. 2022. PMID: 36066827 - The transformation of synaptic to system plasticity in motor output from the sacral cord of the adult mouse.
Jiang MC, Elbasiouny SM, Collins WF 3rd, Heckman CJ. Jiang MC, et al. J Neurophysiol. 2015 Sep;114(3):1987-2004. doi: 10.1152/jn.00337.2015. Epub 2015 Jul 22. J Neurophysiol. 2015. PMID: 26203107 Free PMC article. - Modulation of motoneuron firing by recurrent inhibition in the adult rat in vivo.
Obeidat AZ, Nardelli P, Powers RK, Cope TC. Obeidat AZ, et al. J Neurophysiol. 2014 Nov 1;112(9):2302-15. doi: 10.1152/jn.00358.2014. Epub 2014 Aug 13. J Neurophysiol. 2014. PMID: 25122713 Free PMC article. - Disturbances of motor unit rate modulation are prevalent in muscles of spastic-paretic stroke survivors.
Mottram CJ, Heckman CJ, Powers RK, Rymer WZ, Suresh NL. Mottram CJ, et al. J Neurophysiol. 2014 May;111(10):2017-28. doi: 10.1152/jn.00389.2013. Epub 2014 Feb 26. J Neurophysiol. 2014. PMID: 24572092 Free PMC article.
References
- Ballou EW, Smith WB, Anelli R, Heckman CJ. Measuring dendritic distribution of membrane proteins. J Neurosci Methods. 2006;156:257–266. - PubMed
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. - PubMed
- Bui TV, Cushing S, Dewey D, Fyffe RE, Rose PK. Comparison of the morphological and electrotonic properties of Renshaw cells, Ia inhibitory interneurons, and motoneurons in the cat. J Neurophysiol. 2003;90:2900–2918. - PubMed
- Bui TV, Dewey DE, Fyffe RE, Rose PK. Comparison of the inhibition of Renshaw cells during subthreshold and suprathreshold conditions using anatomically and physiologically realistic models. J Neurophysiol. 2005;94:1688–1698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous