Mononeme: a new secretory organelle in Plasmodium falciparum merozoites identified by localization of rhomboid-1 protease - PubMed (original) (raw)
Mononeme: a new secretory organelle in Plasmodium falciparum merozoites identified by localization of rhomboid-1 protease
Subhash Singh et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Compartmentalization of proteins into subcellular organelles in eukaryotic cells is a fundamental mechanism of regulating complex cellular functions. Many proteins of Plasmodium falciparum merozoites involved in invasion are compartmentalized into apical organelles. We have identified a new merozoite organelle that contains P. falciparum rhomboid-1 (PfROM1), a protease that cleaves the transmembrane regions of proteins involved in invasion. By immunoconfocal microscopy, PfROM1 was localized to a single, thread-like structure on one side of the merozoites that appears to be in close proximity to the subpellicular microtubules. PfROM1 was not found associated with micronemes, rhoptries, or dense granules, the three identified secretory organelles of invasion. Release of merozoites from schizonts resulted in the movement of PfROM1 from the lateral asymmetric localization to the merozoite apical pole and the posterior pole. We have named this single thread-like organelle in merozoites, the mononeme.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
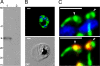
Fig. 1.
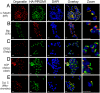
Anti-HA antibodies specifically recognize the HA-PfROM1 protein in transgenic HA-PfROM1 3D7 parasites. (A) Western blot analysis of mature parasite extract probed with anti-HA antibody. Lane 1 shows transgenic HA-PfROM1–3D7 parasite protein extract and lane 2 shows parental 3D7 protein extract. Numbers on the left indicate positions of molecular weight markers in kilodaltons. The arrowhead shows the position of HA-PfROM1 detected in the transgenic parasite extract. (B) IFA analysis of HA-PfROM1 in a developing schizont (4 nuclei stage). (C) Merozoites in a segmenter probed with anti-HA rat mAb 3F10 (green) and the nuclear stain (blue, Upper) or with anti-AMA1 (red, Lower). Note in the individual merozoites anti-HA mAb staining appears to be localized on one side of the nucleus, with characteristic bulbous region (arrowheads) that is toward the apical end of the merozoites. The stalk region runs along one side of the nucleus. (Scale bar, 1 μm.)
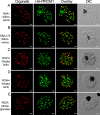
Fig. 2.
HA-PfROM1 is not localized to known apical secretory organelles: rhoptries, micronemes, and dense granules. Staining with antibodies against different secretory organelle markers (red) is shown in the left-hand column. (A) AMA1, a micronemal marker; (B) EBA175RII, another micronemal marker; (C) RAP2, a rhoptry bulb marker; (D) RON4, a rhoptry neck marker; (E) RESA, a dense granule marker. Corresponding costaining with rat anti-HA mAb 3F10 (green) together with the overlay between the red and green channels is shown in the subsequent columns. The right-hand column shows the corresponding differential interference contrast microscopy (DIC) images. (Scale bar, 1 μm.)
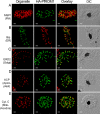
Fig. 3.
HA-PfROM1 does not localize with markers of different known subcellular organelles. Staining with antibodies against different subcellular organelle markers is shown in the left-hand column. (A) MSP1, a merozoite surface marker; (B) Bip, an ER marker; (C) ERD2, a _cis_-Golgi marker; (D) ACP, an apicoplast marker; (E) cytochrome c, a mitochondrial marker. Corresponding costaining with rat anti-HA mAb 3F10 (A and C–E) or mouse anti-HA mAb 2C16 (B) together with the overlay between the red and green channels is shown in the subsequent columns. The right-hand column shows the corresponding DIC images. (Scale bar, 1 μm.)
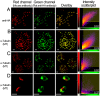
Fig. 4.
Quantitative assessment of the colocalization between HA-PfROM1 and microtubule staining. (A) Staining with mouse anti-HA mAb 2C16 and rat anti-HA mAb 3F10. (B–D) Staining with mouse anti-microtubule mAb DM1A and rat anti-HA mAb of mature segmenters, immature schizonts, and free merozoites, respectively. Scatter 2D plots of voxel intensities in red and green channels observed for the respective pictures are shown in the right-hand column. Intensity threshold in both the channels was automatically determined by excluding dark pixels by using the algorithm in “Coloc” function of the image analysis software, Imaris (Bitplane) as described in ref. . Pearson's correlation coefficient was determined for voxel intensity correlation between the red and green channels and is displayed in the lower-right-hand corner. (Scale bar, 1 μm.)
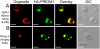
Fig. 5.
Asymmetric organization of organelles confers a lateral polarity in P. falciparum merozoites. Staining with antibodies against different subcellular organelle markers is shown in the left-hand column. (A) α-Tubulin antibodies staining the merozoite subpellicular microtubules. (B) Bip, an ER marker. (C) ERD2, a _cis_-Golgi marker. (D) ACP, an apicoplast marker. (E) Cytochrome c, a mitochondrial marker. Corresponding costaining with rat anti-HA mAb 3F10 (A and C–E) or mouse anti-HA mAb 2C16 (B) together with nuclear staining with DAPI (blue) and an overlay of all channels is shown in subsequent columns. The right-hand column shows a digital zoom of merozoites showing that the red and green staining occurs on the same side of the nucleus. (Scale bar, 1 μm.)
Fig. 6.
HA-PfROM1 is secreted on the merozoite surface and concentrates at the posterior pole of the merozoite. Staining of free merozoites with antibodies against a micronemal marker (A) or with a rhoptry marker at the apical end (B) is shown in the left-hand column (red). Corresponding costaining with rat anti-HA mAb 3F10 (green) together with the overlay between the red and green channels is shown in subsequent columns. The right-hand column shows the corresponding DIC image. Arrow heads point to the merozoite posterior pole. (Scale bar, 1 μm.)
Similar articles
- Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites.
Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Singh S, et al. PLoS Pathog. 2010 Feb 5;6(2):e1000746. doi: 10.1371/journal.ppat.1000746. PLoS Pathog. 2010. PMID: 20140184 Free PMC article. - PfCERLI1 is a conserved rhoptry associated protein essential for Plasmodium falciparum merozoite invasion of erythrocytes.
Liffner B, Frölich S, Heinemann GK, Liu B, Ralph SA, Dixon MWA, Gilberger TW, Wilson DW. Liffner B, et al. Nat Commun. 2020 Mar 16;11(1):1411. doi: 10.1038/s41467-020-15127-w. Nat Commun. 2020. PMID: 32179747 Free PMC article. - Identification and characterization of a novel Plasmodium falciparum merozoite apical protein involved in erythrocyte binding and invasion.
Wickramarachchi T, Devi YS, Mohmmed A, Chauhan VS. Wickramarachchi T, et al. PLoS One. 2008 Mar 5;3(3):e1732. doi: 10.1371/journal.pone.0001732. PLoS One. 2008. PMID: 18320051 Free PMC article. - Extensive differential protein phosphorylation as intraerythrocytic Plasmodium falciparum schizonts develop into extracellular invasive merozoites.
Lasonder E, Green JL, Grainger M, Langsley G, Holder AA. Lasonder E, et al. Proteomics. 2015 Aug;15(15):2716-29. doi: 10.1002/pmic.201400508. Epub 2015 May 19. Proteomics. 2015. PMID: 25886026 Review. - The role of malaria merozoite proteases in red blood cell invasion.
O'Donnell RA, Blackman MJ. O'Donnell RA, et al. Curr Opin Microbiol. 2005 Aug;8(4):422-7. doi: 10.1016/j.mib.2005.06.018. Curr Opin Microbiol. 2005. PMID: 16019257 Review.
Cited by
- Targeting the Plasmodium falciparum proteome and organelles for potential antimalarial drug candidates.
Abugri J, Ayariga J, Sunwiale SS, Wezena CA, Gyamfi JA, Adu-Frimpong M, Agongo G, Dongdem JT, Abugri D, Dinko B. Abugri J, et al. Heliyon. 2022 Aug;8(8):e10390. doi: 10.1016/j.heliyon.2022.e10390. Epub 2022 Aug 24. Heliyon. 2022. PMID: 36033316 Free PMC article. Review. - Downregulation of an Entamoeba histolytica rhomboid protease reveals roles in regulating parasite adhesion and phagocytosis.
Baxt LA, Rastew E, Bracha R, Mirelman D, Singh U. Baxt LA, et al. Eukaryot Cell. 2010 Aug;9(8):1283-93. doi: 10.1128/EC.00015-10. Epub 2010 Jun 25. Eukaryot Cell. 2010. PMID: 20581296 Free PMC article. - Evidence for a Golgi-to-endosome protein sorting pathway in Plasmodium falciparum.
Krai P, Dalal S, Klemba M. Krai P, et al. PLoS One. 2014 Feb 25;9(2):e89771. doi: 10.1371/journal.pone.0089771. eCollection 2014. PLoS One. 2014. PMID: 24587025 Free PMC article. - An essential Aurora-related kinase transiently associates with spindle pole bodies during Plasmodium falciparum erythrocytic schizogony.
Reininger L, Wilkes JM, Bourgade H, Miranda-Saavedra D, Doerig C. Reininger L, et al. Mol Microbiol. 2011 Jan;79(1):205-21. doi: 10.1111/j.1365-2958.2010.07442.x. Epub 2010 Nov 10. Mol Microbiol. 2011. PMID: 21166904 Free PMC article. - Intriguing parasites and intramembrane proteases.
Rawson RB. Rawson RB. Genes Dev. 2008 Jun 15;22(12):1561-6. doi: 10.1101/gad.1686808. Genes Dev. 2008. PMID: 18559471 Free PMC article.
References
- Levine ND, Corliss JO, Cox FE, Deroux G, Grain J, Honigberg BM, Leedale GF, Loeblich AR, III, Lom J, Lynn D, et al. J Protozool. 1980;27:37–58. - PubMed
- Garnham PC, Bird RG, Baker JR. Trans R Soc Trop Med Hyg. 1960;54:274–278. - PubMed
- Aikawa M. Exp Parasitol. 1971;30:284–320. - PubMed
- Adams JH, Hudson DE, Torii M, Ward GE, Wellems TE, Aikawa M, Miller LH. Cell. 1990;63:141–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources