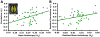Disruption of large-scale brain systems in advanced aging - PubMed (original) (raw)
Disruption of large-scale brain systems in advanced aging
Jessica R Andrews-Hanna et al. Neuron. 2007.
Abstract
Cognitive decline is commonly observed in advanced aging even in the absence of disease. Here we explore the possibility that normal aging is accompanied by disruptive alterations in the coordination of large-scale brain systems that support high-level cognition. In 93 adults aged 18 to 93, we demonstrate that aging is characterized by marked reductions in normally present functional correlations within two higher-order brain systems. Anterior to posterior components within the default network were most severely disrupted with age. Furthermore, correlation reductions were severe in older adults free from Alzheimer's disease (AD) pathology as determined by amyloid imaging, suggesting that functional disruptions were not the result of AD. Instead, reduced correlations were associated with disruptions in white matter integrity and poor cognitive performance across a range of domains. These results suggest that cognitive decline in normal aging arises from functional disruption in the coordination of large-scale brain systems that support cognition.
Figures
Figure 1. Anterior to Posterior Functional Correlations Are Markedly Reduced in Advanced Aging
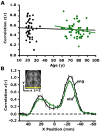
The time course within the medial prefrontal cortex (mPFC) was correlated with the time course within the posterior cingulate/retrosplenial cortex (pC/rsp) for each participant. The resulting _z_-transformed correlation coefficient z(r) for each participant is plotted against age. Data representing young adult participants are colored in black, and those representing older adult participants are colored in green. The black regression line, shown for illustrative purposes only, indicates a strong negative relationship between anterior-posterior functional correlations and age across both groups. The green regression line indicates a negative relationship with age in the older group alone (r = −0.53, p < 0.001). Green data points outlined in black represent PIB-negative individuals. Importantly, their scattered distribution suggests that the age-dependent decline in anterior-posterior functional correlations exists independently of preclinical AD.
Figure 2. Reduced Functional Correlations Observed Anatomically
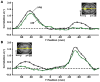
(A) The time course within the mPFC seed region, shown in yellow for only one transverse slice (z = 16) illustrated in the upper right, was correlated with every voxel in the brain. The graph displays the group-averaged _z_-transformed correlation coeffecient, z(r), plotted along a line connecting the center of the mPFC seed and the pC/rsp target regions (shown in [B]), for the anterior-posterior axis. This line is graphically represented in yellow on the transverse slice. The first leftward peak in the graph illustrates that the highest z(r) for both groups is localized to the mPFC seed region. Notice that although the young adult group (black line) exhibits functional correlations with the pC/rsp (indicated by the rightward peak), these correlations are minimally present in the older adult group. (B) A similar analysis was performed for a seed in the pC/rsp, shown for a single transverse slice illustrated in the upper left (z = 28). The yellow line is a graphical representation of a line connecting the center of the seed and the mPFC target (shown in [A]). The group-averaged _z_-transformed correlation coefficients are sampled along the line and plotted against the y position for both groups (black = young; green = old). Error bars = SEM.
Figure 3. Reduced Functional Correlations Are Observed between Multiple Regions within the Large-Scale Brain System
Correlation coefficients between a priori seed and target regions that comprise a large-scale brain system (the default network) are quantified for each group, with values significantly different from zero highlighted in bold (mPFC versus PHC: p < 0.05; all others: p < 0.001). The regions illustrated in yellow include the mPFC, pC/rsp, bilateral lateral parietal cortex (LatPar), bilateral hippocampal formation (HF), and bilateral parahippocampal cortex (PHC). The mean variance computed as the mean of the within-subject variances for each participant’s time course within each region is also listed at the bottom of the table. **Group t test significant at a Bonferroni corrected alpha of 0.005. *Group t test significant at an uncorrected alpha of 0.05.
Figure 4. Whole-Brain Exploratory Analyses Reveal Widespread Correlation Reductions in Aging
Whole-brain analyses of functional correlations between the seed region and each voxel across the entire brain are graphically overlaid on a combined young and old adult anatomical image. (A) For a seed placed in the mPFC, positive correlations with the mPFC time course exceeding a threshold of r = 0.1 are colored in red to yellow and averaged for all young participants (top) and all old participants (middle). A direct comparison between the two groups using the young-old contrast (bottom) highlights voxels at a significance level of p < 0.01. The young group shows higher correlations with many regions comprising the network. (B) The reverse scenario when a seed is placed in the pC/rsp. Functional correlations between the pC/rsp and both the mPFC and the bilateral LatPar, as well as some hint of the HF, decline in old age.
Figure 5. Reduced Functional Correlations Are Present in the Dorsal Attention System
Correlation coefficients between a priori seed and target regions that comprise the dorsal attention system are quantified for each group, with values significantly different from zero highlighted in bold (all: p < 0.05). The regions illustrated in yellow include the intraparietal sulcus (IPS), ventral intraparietal sulcus (vIPS), frontal eye fields (FEF), inferior precentral sulcus (PrCeS), and middle temporal area (MT+). The mean variance, computed as the mean of the within-subject variances for each participant’s time course within each region, is also listed at the bottom of the table. **Group t test significant at a Bonferroni corrected alpha of 0.005. *Group t test significant at a Bonferroni corrected alpha of 0.005. *Significant at an uncorrected alpha of 0.05.
Figure 6. Functional Correlations between the Left and Right Visual Cortex Are Preserved in Aging
(A) The time course was extracted from a region within the right visual cortex (see inset in [B]) and correlated with the time course extracted from the left visual cortex for each participant. The resulting correlation coefficients are plotted against age. The black regression line, used here for illustrative purposes only, suggests that interhemispheric functional correlations in visual cortex remain constant with age. The green regression line illustrates the same effect in the older group alone (r = −0.17, p = 0.22). (B) Exploratory analyses for a seed in the right visual cortex (see inset) are plotted along a line connecting the center of the right and left visual cortex. For each voxel positioned along the x axis, the mean _z_-transformed correlation of all young adults is shown in black, and the mean _z_-transformed correlation of all old adults is shown in green. The rightward _z_-transformed correlation coefficient peak represents functional correlations at the seed (right visual cortex), and the leftward peak represents functional correlations at the target (left visual cortex). Error bars = SEM.
Figure 7. Functional Correlations Relate to White Matter Integrity in Older Adults
(A) Mean anisotropy for a white matter region shown in yellow on one transverse slice was extracted for each participant. The correlation coefficients resulting from correlating the mPFC and pC/rsp time course are plotted against the mean anisotropy (Aσ) (r = 0.39, p < 0.05). (B) A linear regression was performed using the same measures, after controlling for the effect of age on each measure. When doing so, the strength of the relationship between the anterior-posterior functional correlations and white matter integrity remained significant (partial r = 0.33, p < 0.05).
Figure 8. Functional Correlations Relate to Cognitive Performance in Older Adults
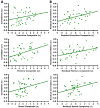
(A) The _z_-transformed correlation coefficients resulting from correlating the mPFC and pC/rsp time course is plotted against the cognitive test scores (converted to _z_-scores) for three different cognitive components (see text for details). The regression lines between the two measures are plotted on each graph. The memory component significantly associates with anterior-posterior functional correlations (executive: r = 0.26, p = 0.11; memory: r = 0.48, p < 0.01; speed: r = 0.24, p = 0.14). (B) A linear regression was performed using the same measures as in (A), after controlling for the effect of age on each measure. When doing so, anterior-posterior functional correlations significantly associated with all three cognitive components (executive: partial r = 0.41, p < 0.01; memory: partial r = 0.40, p < 0.01; speed: r = 0.35, p < 0.05).
Similar articles
- β-Amyloid affects frontal and posterior brain networks in normal aging.
Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. Oh H, et al. Neuroimage. 2011 Feb 1;54(3):1887-95. doi: 10.1016/j.neuroimage.2010.10.027. Epub 2010 Oct 18. Neuroimage. 2011. PMID: 20965254 Free PMC article. - The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans.
Habes M, Pomponio R, Shou H, Doshi J, Mamourian E, Erus G, Nasrallah I, Launer LJ, Rashid T, Bilgel M, Fan Y, Toledo JB, Yaffe K, Sotiras A, Srinivasan D, Espeland M, Masters C, Maruff P, Fripp J, Völzk H, Johnson SC, Morris JC, Albert MS, Miller MI, Bryan RN, Grabe HJ, Resnick SM, Wolk DA, Davatzikos C; iSTAGING consortium, the Preclinical AD consortium, the ADNI, and the CARDIA studies. Habes M, et al. Alzheimers Dement. 2021 Jan;17(1):89-102. doi: 10.1002/alz.12178. Epub 2020 Sep 13. Alzheimers Dement. 2021. PMID: 32920988 Free PMC article. - Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain's default mode network.
Hansen NL, Lauritzen M, Mortensen EL, Osler M, Avlund K, Fagerlund B, Rostrup E. Hansen NL, et al. Hum Brain Mapp. 2014 Sep;35(9):4488-98. doi: 10.1002/hbm.22489. Epub 2014 Feb 27. Hum Brain Mapp. 2014. PMID: 24578157 Free PMC article. - A theory of cognitive control, aging cognition, and neuromodulation.
Braver TS, Barch DM. Braver TS, et al. Neurosci Biobehav Rev. 2002 Nov;26(7):809-17. doi: 10.1016/s0149-7634(02)00067-2. Neurosci Biobehav Rev. 2002. PMID: 12470692 Review. - The adaptive brain: aging and neurocognitive scaffolding.
Park DC, Reuter-Lorenz P. Park DC, et al. Annu Rev Psychol. 2009;60:173-96. doi: 10.1146/annurev.psych.59.103006.093656. Annu Rev Psychol. 2009. PMID: 19035823 Free PMC article. Review.
Cited by
- Mapping the Progression of Atrophy in Early- and Late-Onset Alzheimer's Disease.
Migliaccio R, Agosta F, Possin KL, Canu E, Filippi M, Rabinovici GD, Rosen HJ, Miller BL, Gorno-Tempini ML. Migliaccio R, et al. J Alzheimers Dis. 2015;46(2):351-64. doi: 10.3233/JAD-142292. J Alzheimers Dis. 2015. PMID: 25737041 Free PMC article. - Evaluating the effect of aging on interference resolution with time-varying complex networks analysis.
Ariza P, Solesio-Jofre E, Martínez JH, Pineda-Pardo JA, Niso G, Maestú F, Buldú JM. Ariza P, et al. Front Hum Neurosci. 2015 May 12;9:255. doi: 10.3389/fnhum.2015.00255. eCollection 2015. Front Hum Neurosci. 2015. PMID: 26029079 Free PMC article. - A robust core architecture of functional brain networks supports topological resilience and cognitive performance in middle- and old-aged adults.
Stanford WC, Mucha PJ, Dayan E. Stanford WC, et al. Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2203682119. doi: 10.1073/pnas.2203682119. Epub 2022 Oct 25. Proc Natl Acad Sci U S A. 2022. PMID: 36282912 Free PMC article. - The same, but different: Preserved distractor suppression in old age is implemented through an age-specific reactive ventral fronto-parietal network.
Ashinoff BK, Mayhew SD, Mevorach C. Ashinoff BK, et al. Hum Brain Mapp. 2020 Oct 1;41(14):3938-3955. doi: 10.1002/hbm.25097. Epub 2020 Jun 23. Hum Brain Mapp. 2020. PMID: 32573907 Free PMC article. - Physical activity and brain plasticity in late adulthood.
Erickson KI, Gildengers AG, Butters MA. Erickson KI, et al. Dialogues Clin Neurosci. 2013 Mar;15(1):99-108. doi: 10.31887/DCNS.2013.15.1/kerickson. Dialogues Clin Neurosci. 2013. PMID: 23576893 Free PMC article. Review.
References
- Aizenstein HJ, Clark KA, Butters MA, Cochran J, Stenger VA, Meltzer CC, Reynolds CF, Carter CS. The BOLD hemodynamic response in healthy aging. J Cogn Neurosci. 2004;16:786–793. - PubMed
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. - PubMed
- Bäckman L, Ginovart N, Dixon RA, Wahlin TB, Halldin C, Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157:635–637. - PubMed
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. - PubMed
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG05681/AG/NIA NIH HHS/United States
- AG05886/AG/NIA NIH HHS/United States
- AG00030/AG/NIA NIH HHS/United States
- AG21910/AG/NIA NIH HHS/United States
- F32 AG005886/AG/NIA NIH HHS/United States
- T32 AG000030/AG/NIA NIH HHS/United States
- AG03991/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical