Characterization of adult prostatic progenitor/stem cells exhibiting self-renewal and multilineage differentiation - PubMed (original) (raw)
Characterization of adult prostatic progenitor/stem cells exhibiting self-renewal and multilineage differentiation
Wendy W Barclay et al. Stem Cells. 2008 Mar.
Abstract
Demonstration of the hallmarks of stem cells, self-renewal and multilineage differentiation, is a challenge that has not been met for numerous tissues postulated to possess adult stem cells, including prostate tissue. Using a defined medium, we reproducibly isolated and maintained adult mouse prostatic cells with characteristics of progenitor/stem cells. Clonal populations of cells demonstrated tissue-specific multilineage differentiation by their ability to generate organized prostatic ductal structures in vivo, with luminal and basal cell layers, when grafted under the renal capsules of mice in the presence of fetal rat urogenital mesenchyme. Complete differentiation was demonstrated by the expression and secretion of terminally differentiated prostatic secretory products into the lumens. Self-renewal was demonstrated by serial transplantation of clonal populations that generated fully differentiated ductal structures in vivo. In vitro, undifferentiated cells expressed markers associated with prostate stem cells, including Sca 1 and CD49f, as well as basal cell markers (p63 and cytokeratins 5 and 14) and, at a low level, luminal cell markers (androgen receptor and cytokeratins 8 and 18). When grafted and allowed to differentiate in the presence of fetal urogenital mesenchyme, the cells differentiated into luminal cells and basal cells with more restricted protein expression patterns. These studies are the first to report a reproducible system to assess adult prostatic progenitor/stem cells.
Figures
Figure 1
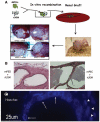
Mixed-population MPECs generate luminal structures in vivo. (A): Schematic of the modified tissue recombination protocol. MPECs (100,000 cells) were mixed with microdissected day 18 fetal rUGM (250,000 cells) that had been cleared of fetal epithelium. The tissue recombinants were grafted under the renal capsules of nude mice and allowed to remain for various periods (3 months in this experiment). Controls were rUGM alone (negative control for rat epithelial contamination) and microdissected mPED + rUGM (positive control). (B): H&E staining of mPED + rUGM control (anterior ducts) and MPEC + rUGM. Original magnification, ×25. (C): Hoechst dye labeling identified punctate nuclear staining (arrows) indicative of the mouse origin of the ductal structures (note diffuse nuclear staining of adjacent rat mesenchymal cells, arrowheads). Abbreviations: MPEC, mouse prostatic epithelial cell; mPED, mouse prostatic epithelial duct; rUGM, rat urogenital mesenchyme; um, micrometer.
Figure 2
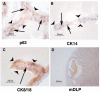
Mouse prostatic epithelial cells differentiate into luminal and basal cells that make prostate secretory products. Sections were prepared from grafts depicted in Figure 1. Sections were probed with antibodies to lineage-specific markers p63 (A), CK14 (B), CK8/18 (C), and mDLP (D). Arrows point to basally located cells; arrowheads point to luminal cells. Abbreviations: CK, cytokeratin; mDLP, mouse dorsolateral secretory protein; um, micrometer.
Figure 3
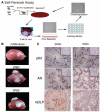
Clonal populations of MPECs form ductal structures. (A): Schematic of experiment. A mixed population of WFU3 cells was inoculated on a 96-well culture dish at 1/2 cell per well. Individual colonies were expanded and grafted with rUGM. (B): H&E sections from grafts from three independent clones. Upper panels are low-magnification images of the slides. (C): Immunohistochemical staining of AR, p63, and mDLP of grafted WFU3 clone 3 cells. Arrows point to basal cells; arrowheads point to luminal cells. Abbreviations: AR, androgen receptor; mDLP, mouse dorsolateral secretory protein; MPEC, mouse prostatic epithelial cell; rUGM, rat urogenital mesenchyme; um, micrometer.
Figure 4
Clonal RbloxP/LoxP mouse prostatic epithelial cells (MPECs) undergo multilineage differentiation in vivo. MPECs were isolated from RbloxP/LoxP animals as previously described [27]. The parental population was subjected to limiting dilution as described for WFU3 MPECs, and clones were grafted with rat urogenital mesenchyme in nude mice. (A): Histology of two independent clones shows luminal structures. (B): Immunodetection of p63 and AR shows a defined basal layer (arrows) and a defined luminal layer (arrowheads). (C): In vitro, the parental cells expressed luminal (CK8 and AR) and basal (CK14 and p63) markers. Insets show 4,6-diamidino-2-phenylindole stain. Original magnification, ×60. Abbreviations: AR, androgen receptor; CK, cytokeratin; um, micrometer.
Figure 5
MPECs retain progenitor/stem cell properties (self-renewal) after serial recombination. (A): Schematic of experiment. Clonally derived Puro-resistant MPECs were grafted with urogenital mesenchyme. After 8 wk in vivo, the Puro-resistant cells were rederived by Puro selection. The rederived population was then subjected to limiting dilution and assayed for multilineage differentiation in vivo by reestablishing MPEC/rUGM recombinants under the renal capsules of nude mice. (B): Gross pictures of grafts after 10 wk in vivo. (C): Immunohistochemical localization of p63, AR, and mDLP. Insets show low magnification of the regions presented at high magnification in the corresponding panels. SRB2 and SR28 are independent clones isolated from separate grafts of WFU3 clone 3 cells as described in Materials and Methods. Abbreviations: AR, androgen receptor; mDLP, mouse dorsolateral secretory protein; MPEC, mouse prostatic epithelial cell; Puro, puromycin; rUGM, rat urogenital mesenchyme; SR, serial recombinant; um, micrometer; wk, weeks.
Figure 6
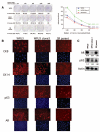
WFU3 clone 3 cells have enhanced clonogenicity and an increased p63 to AR ratio. (A): Clonogenic assays. WFU3, WFU3 clone 3, and SR parent cells were inoculated on 60-mm dishes at the indicated densities and allowed to grow for 4 or 5 days prior to fixation and crystal violet staining as described in Materials and Methods. Left panel shows digital photos of representative dishes after staining. The mean number of colonies ± SD of quadruplicate dishes is depicted below each plate and in the graph at the right. In the graph, lines with the same letter next to them are not statistically significantly different from each other by analysis of variance; p ≤ .05 was considered significant. **, p ≤ .001. Numbers in parentheses are the average colony-forming efficiency ± SD of all inoculation densities, excluding 2,000 cells per dish. (B) Immunofluorescence microscopy of monolayer cultures of WFU3, WFU3 clone 3, and SR parent. Each top panel represents detection with a different primary antibody (indicated). Each bottom panel shows detection of 4,6-diamidino-2-phenylindole fluorescence from same area. Original magnification, ×60. (C): Immunoblot of protein lysates from the indicated cells probed for AR, p63, and β-actin. The numbers under each lane are the ratio of band intensity relative to actin loading. Abbreviations: AR, androgen receptor; CK, cytokeratin; ND, not determined.
Figure 7
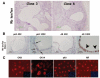
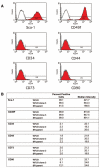
Prostate progenitor/stem cells express stem cell antigen 1 and CD49f. (A): Fluorescence-activated cell sorting analysis of WFU3 cells. The gray line in each graph represents the isotype control fluorescence. The solid line with red fill represents the fluorescence with the specific antibody. M1 is the calculated area of positive reactivity based on the isotype control. (B): Comparison of cell surface marker expression among WFU3, WFU3 clone 3, and SR parent cells. All cells were grown to 70%-80% confluence, and on the same day, cells were harvested and analyzed for marker expression as described in Materials and Methods.
Similar articles
- Cell differentiation lineage in the prostate.
Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Wang Y, et al. Differentiation. 2001 Oct;68(4-5):270-9. doi: 10.1046/j.1432-0436.2001.680414.x. Differentiation. 2001. PMID: 11776479 - Self-renewal and multilineage differentiation in vitro from murine prostate stem cells.
Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Xin L, et al. Stem Cells. 2007 Nov;25(11):2760-9. doi: 10.1634/stemcells.2007-0355. Epub 2007 Jul 19. Stem Cells. 2007. PMID: 17641240 - Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells.
Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Jiang M, et al. Stem Cells. 2010 Feb;28(2):344-56. doi: 10.1002/stem.284. Stem Cells. 2010. PMID: 20020426 Free PMC article. - Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model.
Bonkhoff H, Remberger K. Bonkhoff H, et al. Prostate. 1996 Feb;28(2):98-106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. Prostate. 1996. PMID: 8604398 Review. - Epithelial stem cells of the prostate and their role in cancer progression.
Lukacs RU, Lawson DA, Xin L, Zong Y, Garraway I, Goldstein AS, Memarzadeh S, Witte ON. Lukacs RU, et al. Cold Spring Harb Symp Quant Biol. 2008;73:491-502. doi: 10.1101/sqb.2008.73.012. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022743 Review.
Cited by
- The Role of Integrin α6 (CD49f) in Stem Cells: More than a Conserved Biomarker.
Krebsbach PH, Villa-Diaz LG. Krebsbach PH, et al. Stem Cells Dev. 2017 Aug 1;26(15):1090-1099. doi: 10.1089/scd.2016.0319. Epub 2017 Jun 12. Stem Cells Dev. 2017. PMID: 28494695 Free PMC article. Review. - Prostate cancer stem cells.
Lang SH, Frame FM, Collins AT. Lang SH, et al. J Pathol. 2009 Jan;217(2):299-306. doi: 10.1002/path.2478. J Pathol. 2009. PMID: 19040209 Free PMC article. Review. - Re-epithelialization resulted from prostate basal cells in canine prostatic urethra may represent the ideal healing method after two-micron laser resection of the prostate.
Cao Y, Luo GH, Luo L, Yang XS, Hu JX, Shi H, Huang P, Sun ZL, Xia SJ. Cao Y, et al. Asian J Androl. 2015 Sep-Oct;17(5):831-8. doi: 10.4103/1008-682X.146972. Asian J Androl. 2015. PMID: 25652631 Free PMC article. - Loss of MAP3K7 Sensitizes Prostate Cancer Cells to CDK1/2 Inhibition and DNA Damage by Disrupting Homologous Recombination.
Washino S, Rider LC, Romero L, Jillson LK, Affandi T, Ohm AM, Lam ET, Reyland ME, Costello JC, Cramer SD. Washino S, et al. Mol Cancer Res. 2019 Oct;17(10):1985-1998. doi: 10.1158/1541-7786.MCR-18-1335. Epub 2019 Jul 12. Mol Cancer Res. 2019. PMID: 31300540 Free PMC article. - Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies.
Mimeault M, Mehta PP, Hauke R, Batra SK. Mimeault M, et al. Endocr Rev. 2008 Apr;29(2):234-52. doi: 10.1210/er.2007-0040. Epub 2008 Feb 21. Endocr Rev. 2008. PMID: 18292464 Free PMC article. Review.
References
- Wang Y, Hayward S, Cao M, et al. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. - PubMed
- Magi-Galluzzi C, Loda M. Molecular events in the early phases of prostate carcinogenesis. Eur Urol. 1996;30:167–176. - PubMed
- Aumuller G. Morphologic and endocrine aspects of prostatic function. Prostate. 1983;4:195–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous