CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors - PubMed (original) (raw)
CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors
Yanhua H Huang et al. J Biol Chem. 2008.
Erratum in
- J Biol Chem. 2008 Apr 25;283(17):11860
Abstract
Nucleus accumbens (NAc) medium spiny neurons cycle between two states, a functionally inactive downstate and a functionally active upstate. Here, we show that activation of the transcription factor cAMP-response element-binding protein (CREB), a common molecular response to several drugs of abuse, increases both duration of the upstate and action potential firing during the upstate. This effect of CREB is mediated by enhanced N-methyl-d-aspartate glutamate receptor (NMDAR) function: increased CREB activity increases both NMDAR-mediated synaptic currents and surface level of NMDARs, while inhibition of NMDARs abolishes the effect of CREB on upstate duration. Furthermore, mimicking the effect of CREB by pharmacological enhancement of NMDAR function in the NAc in vivo suppressed novelty- and cocaine-elicited locomotor activity. These findings suggest that by enhancing NMDAR-mediated synaptic transmission, CREB activation promotes the proportion of time NAc neurons spend in the upstate. This effect, along with the CREB enhancement of NAc membrane excitability (Dong, Y., Green, T., Saal, D., Marie, H., Neve, R., Nestler, E. J., and Malenka, R. C. (2006) Nat. Neurosci. 9, 475-477), may counteract drug-induced maladaptations in the NAc and thus ameliorate the addictive state.
Figures
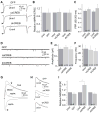
FIGURE 1. CREB influences the upstate of NAc neurons in slice cultures
A, an example neuron filled with Alexa 568 exhibiting typical spiny morphology shown at low (A1) and high (A2,3) magnifications. Neurons expressing caCREB were detected by co-expression of GFP. Calibration bar: 10 _μ_m in A1, 1 _μ_m in A2,3, and 15 _μ_m in A4. B, sample bimodal membrane property of a NAc neuron. C, membrane potential of the bimodal neuron in B was fit by two-Gaussian distribution (green line). D, upstates of NAc neurons were abolished by NBQX. E, whole-cell voltage-clamp recordings showing the occurrence of EPSC bursts in a NAc neuron. Below traces are two segments from the upper trace (indicated by arrows) that were expanded on a slower time scale. F, CREB regulated the upstate duration (F1) and the upstate action potential firing (F2), but not the upstate frequency (F3) or amplitude (F4) in NAc neurons. *, p < 0.05.
FIGURE 2. CREB influences NMDAR EPSCs in NAc neurons
A, sample traces showing AMPAR EPSCs recorded (holding potential −70 mV) from uninfected control neurons and the adjacent manipulated neurons upon stimulation of the same set of presynaptic inputs. B, summary showing that the amplitude of AMPAR EPSCs was not affected by expression of GFP alone, caCREB-GFP, or dnCREB-GFP. C, summary showing that the paired-pulse ratio (PPR) of AMPAR EPSCs was not affected by expression of caCREB or dnCREB. D, sample mEPSCs from GFP-, caCREB-, or dnCREB-expressing NAc neurons. E and F, neither amplitude nor frequency of mEPSCs was affected by expression of caCREB or dnCREB. G, sample traces showing that dual components (mediated by AMPARs and NMDARs as indicated) were recorded from NAc neurons by holding the membrane potential at +40 mV. The dual current at 30 ms (indicated by the upper arrow) after onset was primarily mediated by NMDARs, and, thus, was operationally measured as the amplitude of NMDAR EPSCs. The amplitude of AMPAR EPSCs was measured at the peak (indicated by the lower arrow). H, sample traces showing the dual EPSCs from paired uninfected and manipulated cells. I, summary showing that expression of caCREB significantly increased the amplitude of NMDAR EPSCs in NAc neurons.
FIGURE 3. Activation of CREB up-regulates NMDARs in NAc neurons
A, sample Western blots showing surface and total levels of NR1 subunits in NAc neurons expressing GFP alone, dnCREB, or caCREB. B, summary showing that both the surface level and the ratio of surface to total level of NR1 subunits were increased by caCREB in the NAc, with no significant effect observed on total NR1 levels. *, p < 0.05.
FIGURE 4. NMDARs mediate the CREB effect on upstate duration, but not the upstate action potential firing
A, sample traces from an uninfected NAc neuron showing the bimodal activity before and during application of D-APV. B, summary showing that inhibition of NMDARs reduced the upstate duration of uninfected NAc neurons. C1–2, summaries showing that inhibition of NMDARs abolished the effect of CREB on the upstate duration (C1), but not the upstate action potential firing (C2), in NAc neurons. D, sample voltage traces showing action potential firing elicited by 500 ms of depolarizing current injections (100 and 200 pA) before and during application of D-APV. E, summary showing that activation of CREB up-regulated the evoked action potential firing of NAc neurons, and that inhibition of NMDARs did not influence this CREB effect. *, p < 0.05.
FIGURE 5. Up-regulation of NAc NMDARs suppresses novelty- and cocaine-induced locomotor activity
A, sample traces showing that application of DCS enhanced NMDAR EPSCs in a NAc neuron. B, summary showing that the DCS effect on NMDAR EPSCs was consistently observed in all 11 recorded NAc neurons. C, diagrams showing the injection sites of DCS. The filled dots represent the positions of the tips of cannula that deliver DCS. D, summary showing that rats given intra-NAc injection of DCS did not exhibit significant alteration in their spontaneous locomotor activity. E, summary showing that rats given intra-NAc injection of DCS exhibited significantly reduced novelty-induced horizontal locomotor activity, p < 0.01. F, summary showing that rats given intra-NAc injection of DCS exhibited significantly reduced cocaine-induced locomotor behaviors. (p < 0.01).
Similar articles
- A silent synapse-based mechanism for cocaine-induced locomotor sensitization.
Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas PW, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schlüter OM. Brown TE, et al. J Neurosci. 2011 Jun 1;31(22):8163-74. doi: 10.1523/JNEUROSCI.0016-11.2011. J Neurosci. 2011. PMID: 21632938 Free PMC article. - Morphine-induced inhibition of Ca2+ -dependent d-serine release from astrocytes suppresses excitability of GABAergic neurons in the nucleus accumbens.
Wu J, Zhao R, Guo L, Zhen X. Wu J, et al. Addict Biol. 2017 Sep;22(5):1289-1303. doi: 10.1111/adb.12417. Epub 2016 May 29. Addict Biol. 2017. PMID: 27239019 - NMDA receptor within nucleus accumbens shell regulates propofol self-administration through D1R/ERK/CREB signalling pathway.
Li J, Pan C, Huang B, Qiu J, Jiang C, Dong Z, Li J, Lian Q, Wu B. Li J, et al. Addict Biol. 2024 May;29(5):e13401. doi: 10.1111/adb.13401. Addict Biol. 2024. PMID: 38782631 Free PMC article. - Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".
Nestler EJ. Nestler EJ. Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review. - Roles of nucleus accumbens CREB and dynorphin in dysregulation of motivation.
Muschamp JW, Carlezon WA Jr. Muschamp JW, et al. Cold Spring Harb Perspect Med. 2013 Feb 1;3(2):a012005. doi: 10.1101/cshperspect.a012005. Cold Spring Harb Perspect Med. 2013. PMID: 23293139 Free PMC article. Review.
Cited by
- Transcriptional and epigenetic mechanisms of addiction.
Robison AJ, Nestler EJ. Robison AJ, et al. Nat Rev Neurosci. 2011 Oct 12;12(11):623-37. doi: 10.1038/nrn3111. Nat Rev Neurosci. 2011. PMID: 21989194 Free PMC article. Review. - Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons.
Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y. Ishikawa M, et al. J Neurosci. 2009 May 6;29(18):5820-31. doi: 10.1523/JNEUROSCI.5703-08.2009. J Neurosci. 2009. PMID: 19420249 Free PMC article. - MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212.
Im HI, Hollander JA, Bali P, Kenny PJ. Im HI, et al. Nat Neurosci. 2010 Sep;13(9):1120-7. doi: 10.1038/nn.2615. Epub 2010 Aug 15. Nat Neurosci. 2010. PMID: 20711185 Free PMC article. - The role of D-serine as co-agonist of NMDA receptors in the nucleus accumbens: relevance to cocaine addiction.
D'Ascenzo M, Podda MV, Grassi C. D'Ascenzo M, et al. Front Synaptic Neurosci. 2014 Jul 16;6:16. doi: 10.3389/fnsyn.2014.00016. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25076900 Free PMC article. Review. - Transcriptional mechanisms of drug addiction.
Nestler EJ. Nestler EJ. Clin Psychopharmacol Neurosci. 2012 Dec;10(3):136-43. doi: 10.9758/cpn.2012.10.3.136. Epub 2012 Dec 20. Clin Psychopharmacol Neurosci. 2012. PMID: 23430970 Free PMC article.
References
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. Nat Neurosci. 2006;9:475–477. - PubMed
- Nestler EJ. Nat Rev Neurosci. 2001;2:119–128. - PubMed
- Wilson CJ. Brain Res. 1986;367:201–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources