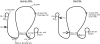What can we learn from rodents about prolactin in humans? - PubMed (original) (raw)
Review
What can we learn from rodents about prolactin in humans?
Nira Ben-Jonathan et al. Endocr Rev. 2008 Feb.
Abstract
Prolactin (PRL) is a 23-kDa protein hormone that binds to a single-span membrane receptor, a member of the cytokine receptor superfamily, and exerts its action via several interacting signaling pathways. PRL is a multifunctional hormone that affects multiple reproductive and metabolic functions and is also involved in tumorigenicity. In addition to being a classical pituitary hormone, PRL in humans is produced by many tissues throughout the body where it acts as a cytokine. The objective of this review is to compare and contrast multiple aspects of PRL, from structure to regulation, and from physiology to pathology in rats, mice, and humans. At each juncture, questions are raised whether, or to what extent, data from rodents are relevant to PRL homeostasis in humans. Most current knowledge on PRL has been obtained from studies with rats and, more recently, from the use of transgenic mice. Although this information is indispensable for understanding PRL in human health and disease, there is sufficient disparity in the control of the production, distribution, and physiological functions of PRL among these species to warrant careful and judicial extrapolation to humans.
Figures
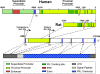
Figure 1
Diagram of the human and rat PRL promoters, the PRL gene, and the human mRNA transcript. Arrows designate transcriptional start sites for the proximal pituitary promoter and the superdistal extrapituitary promoter. The superdistal promoter is unique to humans, and its start site is located 5.8 kb upstream of the pituitary start site. The human and rat proximal promoters differ in size and contain 13 and 8 Pit-1 binding sites, respectively. A functional ERE is present in the rat promoter, whereas its presence in the human proximal promoter is questionable. In both species, the coding region in the pituitary consists of five exons that span approximately 10 kb. Transcription from either promoter produces mRNAs with identical protein coding sequences but differing in the 5′ UTR. Due to the presence of an additional codon in the human gene (1a), extrapituitary PRL mRNA is about 150 bp longer than the pituitary transcript. A signal peptide coding for 28–30 residues lies downstream of the UTR, followed by the PRL transcript.
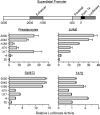
Figure 2
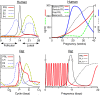
The superdistal PRL promoter (upper panel) and its basal transcriptional activity in several human cell types transfected with various promoter constructs driving a luciferase reporter (lower panel). Cells were transfected with −3000, −2040, −1556, −675, −317, and −4 dPRL truncated constructs. After 72 h, cells were lysed and analyzed for luciferase activity. Transfection efficiency, corrected for Gaussia luciferase, was expressed as fold changes over the PGL3E (3E) plasmid, which was assigned a value of 1. Note the presence of two stimulatory and one inhibitory region in primary breast preadipocytes, with a similar profile seen in SW872 adipocytes. Jurkat lymphocytes do not show the inhibitory region, whereas promoter activity is extremely low in T47D breast cancer cells, suggesting that their PRL expression is not driven by the superdistal promoter (M. McFarland-Mancini and N. Ben-Jonathan, unpublished observations).
Figure 3
Comparison of the human and rat PRL proteins, depicting locations of posttranslational modifications and analog substitution sites. The native protein is composed of 199 and 197 amino acids in humans and rats, respectively, with three disulfide bonds present at similar locations in both species. The main site of glycosylation is at N31 in humans and both T11 and T58 in rats. rPRL is phosphorylated primarily at S177, which is homologous to S179 in humans. Amino acid substitution from S to D mimics phosphorylation (S179D), resulting in an analog that acts as both an agonist and an antagonist. Two other antagonists are G129R, generated by substitution at residue 129 and the Δ1–9G129R double mutant which is also missing the first nine residues. A 16-kDa PRL variant, which acts as an antiangiogenic factor, is formed by cleavage at 145–149, followed by the reduction of the interchain disulfide bond. Two putative heparin binding domains in hPRL are also shown.
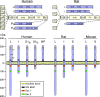
Figure 4
Schematic presentation of the PRLR gene, transcripts, and proteins in humans and rodents. Top panels, PRLR expression is driven by several promoters that code for distinct first exons, E13 and E1N1–N5 in humans and E11–4 in rats. Exons 1, 2, and part of 3 code for the 5′ UTR, whereas the remainder comprise the coding region. Transcripts are alternatively spliced to yield mRNA isoforms of long (L), intermediate (I) and short (S) length. Bottom panel, The PRLR protein consists of an ECD and TM that are identical within species, as well as a cytoplasmic domain of variable length and composition. The length of each isoform is similar in humans and rodents, and common features such as a disulfide bond, WS motif, as well as Box 1 and Box 2 are conserved. Box 2 is not present in some short isoforms. Unique to humans is a soluble PRLR binding protein, which contains only the ECD. Not depicted here are a few additional hPRLR isoforms as well as two other short isoforms in mice. See text for additional explanations.
Figure 5
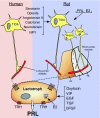
Diagram of the hypothalamo-pituitary system that regulates PRL release in humans and rats. In rats, TIDA neurons originate in the arcuate nucleus and project to the long portal vessels in the median eminence, whereas PHDA neurons, with perikarya located in the periventricular nucleus, terminate in the avascular intermediate lobe (IL). THDA neurons also extend from the arcuate nucleus to both the intermediate lobe and the NL. In humans, there is evidence only for TIDA neurons. Dopamine released from these cells reaches the lactotrophs and inhibits PRL release by acting on D2R. Dopamine synthesis and release in rats is under the control of several brain-derived factors, including stimulators such as angiotensin II, calitonin, neurotensin, and neuropeptide Y (NPY), as well as inhibitors such as serotonin and opioids. PRL itself and estradiol (E2) also affect the hypothalamic dopaminergic systems in rats. In humans, the factors that regulate dopamine production are unknown. PRL synthesis and secretion by rat lactotrophs is directly stimulated by TRH, estrogen, oxytocin, VIP, epidermal growth factor (EGF), TGF, and basic FGF (bFGF), whereas, with the exception of TRH, direct regulators of PRL production in human lactotrophs remain unclear.
Figure 6
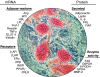
A photograph of fully differentiated human adipocyte LS-14 cell line, as revealed by staining with Oil Red O, surrounded by genes/proteins that have been detected by RT-PCR, ELISA, Western blotting, or enzyme activity. Ang, Angiotensinogen; aP2, adipocyte fatty acid binding protein; Pref-1, preadipocyte factor 1; GLUT4, glucose transporter 4; βAR, β-adrenergic receptor; INSR, insulin receptor; FIAF, fasting-induced adipocyte factor; G6PDH, glucose-6-phosphate dehydrogenase; 6-PGDH, 6-phosphogluconate dehydrogenase; GPDH, glycerol-3-phosphate dehydrogenase. (E. Hugo and N. Ben-Jonathan, unpublished observation.)
Figure 7
Comparison of hormone profiles during the reproductive cycle (left panels) and pregnancy (right panels) in humans and rats. The human menstrual cycle, 28 d in length, consists of a follicular phase, a short ovulatory phase and a luteal phase. In rats, the 4-d estrous cycle is composed of diestrous 2 (D2), proestrous (P), estrous (E) and diestrous 1 (D1). In humans, only a slight increase in PRL occurs during the luteal phase, whereas an estrogen (E2)-induced preovulatory rise in PRL is evident on the afternoon of proestrous in rats, followed by a plateau and an extended termination phase. LH, E2, and progesterone (P4) exhibit a similar secretory profile in the two species. Human pregnancy begins with low PRL levels in the maternal, amniotic, and fetal compartments. Maternal serum PRL rises gradually, from 6–8 wk gestation until term, whereas a steep rise in fetal serum PRL is seen from wk 30 to term. PRL produced by the decidua begins to accumulate in the amniotic fluid at wk 10 and reaches levels as high as 5 μg/ml during midpregnancy, before declining to 500 ng/ml at term. Maternal PL rise concurrently with dPRL but reach a peak of approximately 6 μg/ml before birth. Rodent pregnancy begins with twice daily PRL surges for 10–11 d, followed by suppression of pituitary PRL by the rapidly rising PL-I levels. As PL-I levels drop on d 12, PL-II increases steadily until birth. Pituitary PRL release increases significantly on the day before parturition.
Figure 8
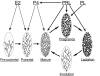
Hormones that regulate mammary gland development and function in mice. Mammary buds form during the early embryonic stage and elongate from birth to puberty. At the onset of estrous cyclicity, the duct system undergoes branching under the influence of both estrogen (E2) and progesterone (P4), the latter being stimulated by PRL. During pregnancy, elevated PRL and PL induce additional ductal branching, as well as the formation and differentiation of alveoli into secretory buds. During lactation, PRL stimulates the production of major milk components. At the termination of lactation, the mammary gland returns to its prepregnancy state through epithelial cell apoptosis (involution) and stromal remodeling and is ready for future pregnancies.
Figure 9
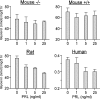
PRL inhibits isoproterenol-stimulated lipolysis in adipose tissue explants from rats and humans, but not mice. Epididymal (mice and rats) and sc abdominal (nonobese woman) explants were incubated with PRL (0, 1, 5, and 25 ng/ml) for 24 h, followed by a 2-h treatment with 100 n
m
isoproterenol. Lipolysis was determined using a colorimetric assay for glycerol release. Data are expressed as nanomoles glycerol/milligram tissue/2 h. Mouse +/+, Wild type; mouse −/−, PRL-deficient. [Modified and redrawn from LaPensee et al. (312).]
Figure 10
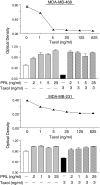
PRL protects breast cancer cells from taxol-induced cytotoxicity. Top panel, MDA-MB-468 cells were treated with increasing doses of taxol (0–625 ng/ml) for 5 d. Cell viability (in all panels) was determined by the MTT assay. The low dose of 5 ng/ml effectively inhibited cell viability by 90%. Second panel, MDA-MB-468 cells were treated with increasing doses of PRL (0.2–25 ng/ml) in the presence or absence of taxol (3 ng/ml) for 5 d. All doses of PRL antagonized taxol-induced cytotoxicity. Third panel, MDA-MB-231 cells were treated with increasing concentrations of taxol (0–625 ng/ml) for 5 d. Cell viability was 10–50% lower in the taxol-treated cells. Bottom panel, MDA-MB-231 cells were exposed to 3 ng/ml of taxol in the presence or absence of PRL (0.2–25 ng/ml) for 5 d. Taxol-induced cell death was not observed when cells were incubated with PRL (E. W. LaPensee and N. Ben-Jonathan, unpublished observations).
Figure 11
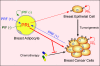
A hypothetical model depicting the role of locally produced PRL in reciprocal stromal-epithelial interactions that promote breast cancer growth. Under normal conditions, PRL production is higher in breast adipocytes than in epithelial cells but is presumably controlled primarily by a PIF. During tumorigenesis, PRF secreted by tumor cells increases PRL secretion from adipocytes, either by antagonizing a PIF or by directly stimulating PRL synthesis. Adipocyte-derived PRL diffuses to the tumor and up-regulates its PRLR expression, increases cell proliferation, and antagonizes chemotherapeutic agents.
Similar articles
- Prolactin (PRL) in adipose tissue: regulation and functions.
Ben-Jonathan N, Hugo E. Ben-Jonathan N, et al. Adv Exp Med Biol. 2015;846:1-35. doi: 10.1007/978-3-319-12114-7_1. Adv Exp Med Biol. 2015. PMID: 25472532 Review. - Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice.
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Bole-Feysot C, et al. Endocr Rev. 1998 Jun;19(3):225-68. doi: 10.1210/edrv.19.3.0334. Endocr Rev. 1998. PMID: 9626554 Review. - From the molecular biology of prolactin and its receptor to the lessons learned from knockout mice models.
Goffin V, Binart N, Clément-Lacroix P, Bouchard B, Bole-Feysot C, Edery M, Lucas BK, Touraine P, Pezet A, Maaskant R, Pichard C, Helloco C, Baran N, Favre H, Bernichtein S, Allamando A, Ormandy C, Kelly PA. Goffin V, et al. Genet Anal. 1999 Nov;15(3-5):189-201. doi: 10.1016/s1050-3862(99)00025-x. Genet Anal. 1999. PMID: 10596761 Review. - Prolactin and the prolactin receptor: new targets of an old hormone.
Harris J, Stanford PM, Oakes SR, Ormandy CJ. Harris J, et al. Ann Med. 2004;36(6):414-25. doi: 10.1080/07853890410033892. Ann Med. 2004. PMID: 15513293 Review. - Mammary gland development and the prolactin receptor.
Binart N, Ormandy CJ, Kelly PA. Binart N, et al. Adv Exp Med Biol. 2000;480:85-92. doi: 10.1007/0-306-46832-8_10. Adv Exp Med Biol. 2000. PMID: 10959413 Review.
Cited by
- 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis.
Grattan DR. Grattan DR. J Endocrinol. 2015 Aug;226(2):T101-22. doi: 10.1530/JOE-15-0213. Epub 2015 Jun 22. J Endocrinol. 2015. PMID: 26101377 Free PMC article. Review. - Does the rate of orthodontic tooth movement change during pregnancy and lactation? A systematic review of the evidence from animal studies.
Omar M, Kaklamanos EG. Omar M, et al. BMC Oral Health. 2020 Aug 27;20(1):237. doi: 10.1186/s12903-020-01223-2. BMC Oral Health. 2020. PMID: 32854696 Free PMC article. - Inhibition of MAPK by prolactin signaling through the short form of its receptor in the ovary and decidua: involvement of a novel phosphatase.
Devi YS, Seibold AM, Shehu A, Maizels E, Halperin J, Le J, Binart N, Bao L, Gibori G. Devi YS, et al. J Biol Chem. 2011 Mar 4;286(9):7609-18. doi: 10.1074/jbc.M110.166603. Epub 2011 Jan 3. J Biol Chem. 2011. PMID: 21199871 Free PMC article. - Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons.
Belugin S, Diogenes AR, Patil MJ, Ginsburg E, Henry MA, Akopian AN. Belugin S, et al. J Biol Chem. 2013 Nov 29;288(48):34943-55. doi: 10.1074/jbc.M113.486571. Epub 2013 Oct 18. J Biol Chem. 2013. PMID: 24142695 Free PMC article. - Effect of prolactin on penile erection: a cross-sectional study.
Xu ZH, Pan D, Liu TY, Yuan MZ, Zhang JY, Jiang S, Wang XS, Guan Y, Zhao ST. Xu ZH, et al. Asian J Androl. 2019 Nov-Dec;21(6):587-591. doi: 10.4103/aja.aja_22_19. Asian J Androl. 2019. PMID: 31044754 Free PMC article.
References
- Forsyth IA, Wallis M 2002 Growth hormone and prolactin–molecular and functional evolution. J Mammary Gland Biol Neoplasia 7:291–312 - PubMed
- Handwerger S, Freemark M 2000 The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab 13:343–356 - PubMed
- Goffin V, Binart N, Touraine P, Kelly PA 2002 Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67 - PubMed
- Prigent-Tessier A, Tessier C, Hirosawa-Takamori M, Boyer C, Ferguson-Gottschall S, Gibori G 1999 Rat decidual prolactin. Identification, molecular cloning, and characterization. J Biol Chem 274:37982–37989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 BC005725/ImNIH/Intramural NIH HHS/United States
- T32 CA117846/CA/NCI NIH HHS/United States
- ES012212/ES/NIEHS NIH HHS/United States
- CA096613/CA/NCI NIH HHS/United States
- R01 ES012212/ES/NIEHS NIH HHS/United States
- R01 CA096613/CA/NCI NIH HHS/United States
- T32 ES007250/ES/NIEHS NIH HHS/United States
- T32-ES 007250/ES/NIEHS NIH HHS/United States
- T32-CA 117846/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources