Lipids revert inert Abeta amyloid fibrils to neurotoxic protofibrils that affect learning in mice - PubMed (original) (raw)
Comparative Study
. 2008 Jan 9;27(1):224-33.
doi: 10.1038/sj.emboj.7601953. Epub 2007 Dec 6.
Inna Kuperstein, Hannah Wilkinson, Elke Maes, Mieke Vanbrabant, Wim Jonckheere, Patrick Van Gelder, Dieter Hartmann, Rudi D'Hooge, Bart De Strooper, Joost Schymkowitz, Frederic Rousseau
Affiliations
- PMID: 18059472
- PMCID: PMC2206134
- DOI: 10.1038/sj.emboj.7601953
Comparative Study
Lipids revert inert Abeta amyloid fibrils to neurotoxic protofibrils that affect learning in mice
Ivo Cristiano Martins et al. EMBO J. 2008.
Abstract
Although soluble oligomeric and protofibrillar assemblies of Abeta-amyloid peptide cause synaptotoxicity and potentially contribute to Alzheimer's disease (AD), the role of mature Abeta-fibrils in the amyloid plaques remains controversial. A widely held view in the field suggests that the fibrillization reaction proceeds 'forward' in a near-irreversible manner from the monomeric Abeta peptide through toxic protofibrillar intermediates, which subsequently mature into biologically inert amyloid fibrils that are found in plaques. Here, we show that natural lipids destabilize and rapidly resolubilize mature Abeta amyloid fibers. Interestingly, the equilibrium is not reversed toward monomeric Abeta but rather toward soluble amyloid protofibrils. We characterized these 'backward' Abeta protofibrils generated from mature Abeta fibers and compared them with previously identified 'forward' Abeta protofibrils obtained from the aggregation of fresh Abeta monomers. We find that backward protofibrils are biochemically and biophysically very similar to forward protofibrils: they consist of a wide range of molecular masses, are toxic to primary neurons and cause memory impairment and tau phosphorylation in mouse. In addition, they diffuse rapidly through the brain into areas relevant to AD. Our findings imply that amyloid plaques are potentially major sources of soluble toxic Abeta-aggregates that could readily be activated by exposure to biological lipids.
Figures
Figure 1
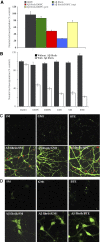
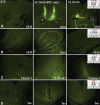
Lipid-derived Aβ protofibrils cause neurotoxicity and cell death in primary hippocampal neurons. Inert Aβ fibrils (250 mg ml−1, 50 μM) were incubated with DOPC liposomes (2.5 mg ml−1) overnight while shaking and subsequently added in a 1:10 dilution (5 μM and 0.25 mg ml−1 final concentrations of fibrils and lipids, respectively) to primary cultures of hippocampal neurons cultured for 18 DIV (days in vitro). (A) Neutral red incorporation by neurons after treatment with lipids (0.25 mg ml−1 final concentration), Aβ fibrils (5 μM final concentration) or lipid/Aβ fibrils during 48 h was assayed. DOPC liposomes alone (black), Aβ fibrils alone (green), Aβ fibril/DOPC (red), Aβfibril/DOPC soluble fraction obtained by extensive centrifugation (blue) or resuspended Aβfibril/DOPC insoluble pelleted fraction (yellow) are shown. Values are % of control±s.e.m., P<0.002, of three independent experiments performed in triplicate. (B) Neutral red incorporation by neurons treated for 24 h with various lipid mixtures (black) or with soluble fraction from Aβ fibril/lipid mixtures (white) is shown (concentrations as above). DOPC, dioleyl phosphatidylcholine; DMPG, dioleyl phosphatidylglycerol, GM1, ganglioside; SM, sphingomyelin (SM); BTE, brain total extract. Values are % of control±s.e.m., P<0.006, of three independent experiments performed in triplicate. Aβ fibrils (5 μM final concentration) or lipid mixtures (0.25 mg ml−1 final concentration) alone had no effect on neutral red incorporation. (C) AnnexinV/propidium iodide (PI) staining of primary neurons. Fluorescence microscopy images of Annexin V (green) and PI (red) staining of hippocampal neurons cultured for 18 DIV treated with GM1, SM and BTE liposomes and the soluble fraction of the same Aβ fibrils/lipid mixtures in the concentrations mentioned above are shown. Incubation with 5 μM of Aβ fibrils alone did not affect this staining (not shown). (D) Cleaved caspase-3 staining. Fluorescence microscopy images of cleaved caspase-3 staining of hippocampal neurons cultured for 18 DIV treated with SM, GM1 and BTE liposomes and soluble fractions of Aβ fibrils/lipid mixtures as mentioned above are shown. Incubation with 5 μM of Aβ fibrils alone did not affect this staining (not shown). Results reveal apoptotic cell death induced by soluble fractions of Aβ fibrils/lipid mixtures but not by Aβ fibrils or lipids alone.
Figure 2
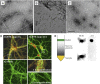
Lipids induce disassembly of mature Aβ42 amyloid fibrils into soluble protofibrils. (A) Electron microscopy images showing mature Aβ42 amyloid fibrils alone. (B) Aβ42 amyloid fibrils were mixed with DOPC liposomes and the pellet was harvested using centrifugation; the electron micrograph reveal strongly intertwined lipids and amyloid fibrils as well as fragmented amyloid material. (C) Electron micrograph of the soluble fraction of the previous image, which contains small oligomeric fragments. The black bar on images A, B and C indicates a size of 200 nm. (D) Protofibrils detection by oligomer-specific A11 antibody. Soluble fractions from Aβ fibrils/BTE mixtures display decorating punctuate-like hippocampal neurons staining detected by the oligomer-specific A11 antibody (red) versus actin staining (green) revealing binding of oligomers to the cell surface and intracellular localization at later time points. (E) ‘Floating assay' by ultracentrifugation reveals Alzheimer's Aβ42 oligomers in association with liposomes in amyloid/lipid mixtures (250 μg ml−1 peptide, 2.5 mg ml−1 lipid). After centrifugation for 1 h at 150 000 g, liposomes are in the top fraction, whereas protein aggregates are expected in the pellet. As 6E10 immunostaining indicates, some Aβ42 material is indeed removed to the pellet, but a significant amount is transported to the top fraction in association with the lipids. This fraction is equally recognized by the oligomer-specific antibody A11, consistent with fibril disassembly, whereas the pellet fraction is not recognized by A11 and most likely contains intact fibrils.
Figure 3
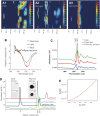
Biophysical characterization of lipid-induced protofibrils. (A) DLS spectra of mature Aβ42 fibrils (A1), fibril/lipid mixtures (A2) and pure liposome preparations (A3, DOPC). Apparent hydrodynamic radii are indicated on the x axis (logarithmic scale), the y axis shows the evolution of the signal during the experiment (20 s) and the color code indicates a relative intensity scale between 0 and 1. The liposome spectrum (A3, 2.5 mg ml−1 lipid concentration) reveals strong monodispersity in the sample with an average hydrodynamic radius just above 100 nm, whereas the amyloid fibril sample (A1, 50 μM peptide concentration) contains a wider range of molecular sizes, ranging from 5 to 100 μm approximately. The spectrum of the amyloid–lipid mixture (A2, 50 μM peptide concentration and 2.5 mg ml−1 lipid concentration) shows a complete loss of signal for hydrodynamic radii above 5 μm. The majority of the signal shows strong heterogeneity with sizes ranging from 100 nm and 1 μm, consistent with the disassembly of amyloid fibrils into smaller species. (B) Far UV CD spectroscopy gives information on the secondary structure of material in solution. The spectra of Aβ42 amyloid fibrils in isolation (50 μM) and in the presence of liposomes (2.5 mg ml−1) containing DMPG display a marked increase in the intensity of the spectrum around 220 nm, while the overall shape of the spectrum is constant. This is consistent with an increase in the amount of soluble material rich in β-sheet content. Similar results were obtained with other lipids (not shown). (C) FTIR yields information on the secondary structure of both the soluble and insoluble material in the sample. The spectra of mature Aβ42 fibrils (50 μM) and fibril/lipid mixtures (BTE, 2.5 mg ml−1) show a strong cross-β signal at 1623 cm−1, consistent with a comparable β-sheet content for amyloid fibrils and protofibrils. However, the difference spectrum reveals an additional band at 1647, corresponding to the formation of random coil in the amyloid/lipid mixture, consistent with partial fibril disassembly. (D) SEC on a GEHealthcare S75/HR10 column of ‘forward' (blue line) and lipid-induced (‘backward') oligomers (green line). For each sample, 200 μl of an amyloid–lipid mixture containing 250 μg of Aβ42 peptide and 2.5 mg ml−1 lipid were injected. Notice peak elutions at 15.8 ml and 16.9 ml, respectively. Upon 0.1% SDS treatment, backward oligomers elute at the same position as forward oligomers, whereas lipids elute in the void volume (black line). For comparison, an elution trace for monomeric Aβ42 is also shown (red line, elution peak at 21 ml). Elution profiles of amyloid fibril samples show no noticeable peaks. Inset show immunostaining of the lipid fraction and the Aβ42/lipid-induced oligomer fraction of the black profile using the antibody A11, which specifically detects oligomers, and the 6E10 mAb, which is specific for Aβ. (E) Eighteen-angles SLS, placed inline with the SEC system, allows to determine molecular mass in an absolute manner that does not depend on interactions with column matrix (as is the case in SEC) or assumed molecular shape (as is the case in DLS). The 15.8 ml peak shown in Figure 3D was analyzed in this manner. The Zimm plot (see Materials and methods) in Figure 3E shows the clear nonlinear dependence of the light scattering intensity with scattering angle, consistent with a radius of gyration larger than 80 nm. The red curve indicates a fit to a quadratic equation (R-squared value >0.9). The molecular masses obtained in this manner vary throughout the peak, in agreement with the heterogeneity observed in DLS and electron microscopy and consistent with a total loss of size-sorting effects from the column due to nonspecific matrix interactions. Masses obtained vary from 80 to 500 kDa throughout the peak.
Figure 4
Morphology and toxicity of forward protofibrils. (A) Electron micrograph of 48 h aged solution of freshly dissolved Aβ42 reveals a mixture of protofibrillar intermediates and the first appearance of some amyloid fibrils. (B) Neutral red incorporation by hippocampal neurons treated with monomers (5 μM), mature Aβ fibrils (5 μM), forward oligomers generated from monomers during 48 h aggregation (5 μM) and soluble fractions of Aβ fibrils/lipid mixtures at 5 μM and 0.25 mg ml−1 final concentrations of fibrils and lipids, respectively (termed as ‘backward lipid-derived protofibrils', see text). Values are % of control±s.e.m., P<0.008, of two independent experiments performed in quadruplicates.
Figure 5
Lipid-derived Aβ protofibrils diffuse rapidly in the brain of mice. (A) Detection of intraventricularly injected Aβ preparations in the mouse brain. Inert Aβ fibrils (250 mg ml−1, 50 μM) were incubated with BTE liposomes (2.5 mg ml−1) overnight while shaking and then centrifuged as indicated above. A volume of 3 μl of Aβ fibrils/BTE mixtures soluble fractions from these preparations were injected bilaterally into the lateral ventricles. The same volume of mature fibrils (50 μM) or lipid preparations (2.5 mg ml−1) alone were injected into two control groups. After 1.5 h, mice were killed, and staining with the Aβ-specific mAb 6E10 antibody revealed strongly pronounced Aβ staining along the needle track and in the ventricles in fibrils/BTE mixtures soluble fractions-injected brains (A, middle panel). Similar staining in the ventricles is observed when Aβ fibrils alone are injected, but much less staining is observed along the needle tracks (A, right panel), indicating partial perfusion of soluble Aβ species, but not mature fibrils in the brain tissue. Brains of mice injected with BTE lipid alone did not show Aβ-specific staining (A, left panel). (B) Distribution of intraventricularly injected Aβ preparations in the hippocampus of mouse brain. After 1.5 h of injection, strong and specific staining of the hippocampal area is observed with mAb 6E10 when fibrils/BTE mixture-soluble fractions are injected (B, middle panel). Such staining is not observed with lipids (B, left panel) or Aβ fibrils injected alone (A, right panel). (C) Cleaved Caspase-3 detection in mouse brain injected with Aβ preparations. Injection of fibrils/BTE mixture-soluble fraction for 24 h caused cleaved caspase-3 (cell signaling) activation in frontal cortex area (C, middle panel). Some caspase-3 staining limited to the area around ventricles and needle track is seen with fibril injections (A, right panel). Such staining is not observed with lipids (B, left panel). (D) Phosphorylated tau detection in mouse brain injected with Aβ preparations. Phosphorylated tau staining (AT8 mAb) was detected in the same frontal cortex areas as for cleaved caspase-3 in brains injected with fibrils/BTE mixture-soluble fraction and fibrils. Data indicate that apoptotic markers such as caspase-3 activation and tau phosphorylation are associated with distribution of soluble Aβ in brains injected with fibrils/BTE mixture-soluble fraction, but less in fibrils-injected brains.
Figure 6
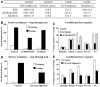
Lipid-derived and forward Aβ protofibrils cause learning and memory impairments in mice. A volume of 3 μl of Aβ fibrils/BTE mixtures soluble fractions, mature fibrils or lipids were injected bilaterally into the lateral ventricles under local anesthesia as indicated in Figure 5. After 1.5 h, mice were evaluated in several behavioral assays. (A) Open-field recording of mice injected with backward protofibrils. The table displays total path length covered, velocity, frequencies of center visits and time spent in center. Data reveal that injection of Aβ fibrils/BTE mixture-soluble fractions caused increased velocity and path length covered and increased frequency of visits to the center, but declined time spent in the center. Overall, these indicate hyperactivation and hyperactivity. This effect is not observed in groups injected with mature fibrils and lipids alone (values are mean±s.e.m., P<0.007, _n_=20, 21 and 13 for BTE, Aβ fibrils and Aβ fibrils/BTE, respectively). (B) Passive avoidance test of mice injected with backward protofibrils. Light–dark step through test showed latency of entrance during the training accompanied with electrical shock (white) and during the testing 24 h later (black). Injection of Aβ fibrils/BTE mixture-soluble fractions 1.5 h before the shock impaired memory in contrast to groups injected with mature fibrils and lipids alone (values are latency mean±s.e.m., P<0.04, _n_=15 for each experimental group). (C) Contextual fear response test of mice injected with backward protofibrils. Mice groups injected with BTE (white), Aβ fibrils (gray) and Aβ fibrils/BTE mixture-soluble fractions (black) were provided with an electrical stimulus accompanied by an auditory conditioned stimulus 1.5 h after the injection. Memorization of this combination is tested after 24 h by the freezing behavior of the mice exposed to the similar context or to the auditory conditioning stimulus. Data show decreased freezing events (indicating memory disturbances) during exposure to context and to conditioned stimulus in the group injected with Aβ fibrils/BTE mixture-soluble fractions in contrast to groups injected with mature fibrils and lipids alone (values are % freezing mean±s.e.m., P<0.005, _n_=20, 21 and 13 for BTE, Aβ fibrils and Aβ fibrils/BTE, respectively). (D) Passive avoidance test of mice injected with forward protofibrils. Light–dark step through test showing latency of entrance during the training accompanied with electrical shock (weight) and during the testing 24 h later (black) (values are latency mean±s.e.m., P<0.001, _n_=10 for oligomers group and 6 for control group). (E) Contextual fear response test of mice injected with forward protofibrils. Freezing response during the test blocks in groups injected with vehicle (weight) and forward Aβ oligomers (black) 1.5 h after the injection (values are % freezing mean±s.e.m., P<0.005, _n_=12 for oligomers group and 6 for control group).
Similar articles
- Lecanemab demonstrates highly selective binding to Aβ protofibrils isolated from Alzheimer's disease brains.
Johannesson M, Söderberg L, Zachrisson O, Fritz N, Kylefjord H, Gkanatsiou E, Button E, Svensson AS, Rachalski A, Nygren P, Osswald G, Lannfelt L, Möller C. Johannesson M, et al. Mol Cell Neurosci. 2024 Sep;130:103949. doi: 10.1016/j.mcn.2024.103949. Epub 2024 Jun 20. Mol Cell Neurosci. 2024. PMID: 38906341 - Structural properties of Abeta protofibrils stabilized by a small molecule.
Williams AD, Sega M, Chen M, Kheterpal I, Geva M, Berthelier V, Kaleta DT, Cook KD, Wetzel R. Williams AD, et al. Proc Natl Acad Sci U S A. 2005 May 17;102(20):7115-20. doi: 10.1073/pnas.0408582102. Epub 2005 May 9. Proc Natl Acad Sci U S A. 2005. PMID: 15883377 Free PMC article. - Isolated amyloid-β(1-42) protofibrils, but not isolated fibrils, are robust stimulators of microglia.
Paranjape GS, Gouwens LK, Osborn DC, Nichols MR. Paranjape GS, et al. ACS Chem Neurosci. 2012 Apr 18;3(4):302-11. doi: 10.1021/cn2001238. Epub 2012 Jan 9. ACS Chem Neurosci. 2012. PMID: 22860196 Free PMC article. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Protofibrils of Amyloid-β are Important Targets of a Disease-Modifying Approach for Alzheimer's Disease.
Ono K, Tsuji M. Ono K, et al. Int J Mol Sci. 2020 Jan 31;21(3):952. doi: 10.3390/ijms21030952. Int J Mol Sci. 2020. PMID: 32023927 Free PMC article. Review.
Cited by
- Potential mechanisms and implications for the formation of tau oligomeric strains.
Gerson JE, Mudher A, Kayed R. Gerson JE, et al. Crit Rev Biochem Mol Biol. 2016 Nov/Dec;51(6):482-496. doi: 10.1080/10409238.2016.1226251. Epub 2016 Sep 21. Crit Rev Biochem Mol Biol. 2016. PMID: 27650389 Free PMC article. Review. - Stable size distribution of amyloid plaques over the course of Alzheimer disease.
Serrano-Pozo A, Mielke ML, Muzitansky A, Gómez-Isla T, Growdon JH, Bacskai BJ, Betensky RA, Frosch MP, Hyman BT. Serrano-Pozo A, et al. J Neuropathol Exp Neurol. 2012 Aug;71(8):694-701. doi: 10.1097/NEN.0b013e31825e77de. J Neuropathol Exp Neurol. 2012. PMID: 22805771 Free PMC article. - Structure and Function of Alzheimer's Amyloid βeta Proteins from Monomer to Fibrils: A Mini Review.
Agrawal N, Skelton AA. Agrawal N, et al. Protein J. 2019 Aug;38(4):425-434. doi: 10.1007/s10930-019-09854-3. Protein J. 2019. PMID: 31325011 Review. - A yeast toxic mutant of HET-s((218-289)) prion displays alternative intermediates of amyloidogenesis.
Berthelot K, Lecomte S, Géan J, Immel F, Cullin C. Berthelot K, et al. Biophys J. 2010 Aug 9;99(4):1239-46. doi: 10.1016/j.bpj.2010.06.015. Biophys J. 2010. PMID: 20713008 Free PMC article. - Estrogen stimulates degradation of beta-amyloid peptide by up-regulating neprilysin.
Liang K, Yang L, Yin C, Xiao Z, Zhang J, Liu Y, Huang J. Liang K, et al. J Biol Chem. 2010 Jan 8;285(2):935-42. doi: 10.1074/jbc.M109.051664. Epub 2009 Nov 6. J Biol Chem. 2010. PMID: 19897485 Free PMC article.
References
- Aksenov MY, Aksenova MV, Butterfield DA, Hensley K, Vigo-Pelfrey C, Carney JM (1996) Glutamine synthetase-induced enhancement of beta-amyloid peptide A beta (1–40) neurotoxicity accompanied by abrogation of fibril formation and A beta fragmentation. J Neurochem 66: 2050–2056 - PubMed
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H (2005) Globular amyloid beta-peptide oligomer—a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem 95: 834–847 - PubMed
- Bitan G, Teplow DB (2004) Rapid photochemical cross-linking—a new tool for studies of metastable, amyloidogenic protein assemblies. Acc Chem Res 37: 357–364 - PubMed
- Bitan G, Vollers SS, Teplow DB (2003) Elucidation of primary structure elements controlling early amyloid beta-protein oligomerization. J Biol Chem 278: 34882–34889 - PubMed
- Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, Hawkins PN, Dobson CM, Radford SE, Blake CC, Pepys MB (1997) Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385: 787–793 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources