Assembly, organization, and function of the COPII coat - PubMed (original) (raw)
Review
Assembly, organization, and function of the COPII coat
Helen Hughes et al. Histochem Cell Biol. 2008 Feb.
Abstract
A full mechanistic understanding of how secretory cargo proteins are exported from the endoplasmic reticulum for passage through the early secretory pathway is essential for us to comprehend how cells are organized, maintain compartment identity, as well as how they selectively secrete proteins and other macromolecules to the extracellular space. This process depends on the function of a multi-subunit complex, the COPII coat. Here we describe progress towards a full mechanistic understanding of COPII coat function, including the latest findings in this area. Much of our understanding of how COPII functions and is regulated comes from studies of yeast genetics, biochemical reconstitution and single cell microscopy. New developments arising from clinical cases and model organism biology and genetics enable us to gain far greater insight in to the role of membrane traffic in the context of a whole organism as well as during embryogenesis and development. A significant outcome of such a full understanding is to reveal how the machinery and processes of membrane trafficking through the early secretory pathway fail in disease states.
Figures
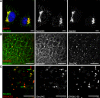
Fig. 1
Localization of COPII in mammalian cells. a Sec24C (COPII, green) and giantin (a marker of the cis-/medial-Golgi, red) in HeLa cells. Note the close proximity of the two structures in the juxtanuclear region results in apparent colocalization and yellow colouring in the merge. Bar 10 μm. b Sec24C (red) and calnexin (green) show the presence of ERES on ER membranes. Bar 10 μm. c Immunofluorescence labelling of methanol fixed HeLa cell shows the close apposition but distinct localization of Sec24C (COPII, green) and ERGIC-53 (red, a marker of the ER-Golgi intermediate compartment). Arrowheads highlight the clear separation of the two structures. Bar 5 μm. Individual green and red channels are shown alongside the merge for clarity
Fig. 2
Schematic model for COPII–COPI coupling. The model shows the assembly and putative coupling of COPI and COPII function in mammalian cells. Starting from the top, the model shows the early stages of assembly of the COPII complex, dependent on Sar1 and Sec16 function. The blue box indicates events at the ERES and the protein components are labelled as detailed in the legend. This model is based on published work and includes known interactions. What is unclear is precisely how many of these interactions occur together i.e. just how large the complexes/subcomplexes can get. For example, we have no real idea whether there is a point at which Sec16 and COPI can be present on the same membrane/vesicle. Sec16 is drawn as part of the budded vesicle as the true fate of Sec16 during the COPII assembly/budding process remains unclear. At the stage of nascent VTC/tethering complex assembly (light blue) we have suggested that Sec13/31 dissociates. This is based on observations linking Sec23/24 to multiple important components that operate post budding such as TRAPPI and dynactin. The nascent VTC stage (light yellow) precedes complete loss of the COPII components, after which one is left with a COPI-coated VTC
Fig. 3
Sequential assembly of the COPII coat structure. Following activation of Sar1-GDP to Sar1-GTP by the membrane-bound GEF Sec12, Sec23/24 is recruited to the ER membrane. Sec23/24 provides the major cargo binding function of the coat. Sec12 could be geometrically excluded from ERES because of membrane curvature. The final outer layer coat components Sec13/13 are recruited just prior to scission events, forming a vesicle. The minimal cage structure is shown; vesicle size would be dictated by cargo incorporation and potentially by other factors. These are schematic illustrations generated using Autodesk Maya 8.0 and are not intended as accurate rendering of the precise atomic coordinates from crystallographic data
Fig. 4
Sec16 spatially restricts assembly of the COPII coat. a Outer coat components Sec13/31 form cuboctahedrons in their minimal state. The proline-rich region of Sec13 contacts Sec23/24 and Sar1 GTP stabilizing contacts between the inner and outer coat complexes. This also has the capacity to position Sec23/24 underneath the edges of the cage making the membrane surface (and associated proteins) more accessible. These are schematic illustrations generated using Autodesk Maya 8.0 and are not intended as accurate rendering of the precise atomic coordinates from crystallographic data
Similar articles
- ER-to-Golgi transport: COP I and COP II function (Review).
Duden R. Duden R. Mol Membr Biol. 2003 Jul-Sep;20(3):197-207. doi: 10.1080/0968768031000122548. Mol Membr Biol. 2003. PMID: 12893528 Review. - Traffic COPs of the early secretory pathway.
Barlowe C. Barlowe C. Traffic. 2000 May;1(5):371-7. doi: 10.1034/j.1600-0854.2000.010501.x. Traffic. 2000. PMID: 11208122 Review. - Vesicle-mediated export from the ER: COPII coat function and regulation.
D'Arcangelo JG, Stahmer KR, Miller EA. D'Arcangelo JG, et al. Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review. - Auxilin facilitates membrane traffic in the early secretory pathway.
Ding J, Segarra VA, Chen S, Cai H, Lemmon SK, Ferro-Novick S. Ding J, et al. Mol Biol Cell. 2016 Jan 1;27(1):127-36. doi: 10.1091/mbc.E15-09-0631. Epub 2015 Nov 4. Mol Biol Cell. 2016. PMID: 26538028 Free PMC article. - The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the golgi.
Lord C, Ferro-Novick S, Miller EA. Lord C, et al. Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a013367. doi: 10.1101/cshperspect.a013367. Cold Spring Harb Perspect Biol. 2013. PMID: 23378591 Free PMC article. Review.
Cited by
- Early stages of retinal development depend on Sec13 function.
Schmidt K, Cavodeassi F, Feng Y, Stephens DJ. Schmidt K, et al. Biol Open. 2013 Mar 15;2(3):256-66. doi: 10.1242/bio.20133251. Epub 2013 Jan 10. Biol Open. 2013. PMID: 23519012 Free PMC article. - Combinatorial multivalent interactions drive cooperative assembly of the COPII coat.
Stancheva VG, Li XH, Hutchings J, Gomez-Navarro N, Santhanam B, Babu MM, Zanetti G, Miller EA. Stancheva VG, et al. J Cell Biol. 2020 Nov 2;219(11):e202007135. doi: 10.1083/jcb.202007135. J Cell Biol. 2020. PMID: 32997735 Free PMC article. - A genome-wide analysis of coatomer protein (COP) subunits of apicomplexan parasites and their evolutionary relationships.
Kibria KMK, Ferdous J, Sardar R, Panda A, Gupta D, Mohmmed A, Malhotra P. Kibria KMK, et al. BMC Genomics. 2019 Jan 31;20(1):98. doi: 10.1186/s12864-019-5463-1. BMC Genomics. 2019. PMID: 30704415 Free PMC article. - Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif.
Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Ivan V, et al. Mol Biol Cell. 2008 Oct;19(10):4352-65. doi: 10.1091/mbc.e08-03-0246. Epub 2008 Jul 9. Mol Biol Cell. 2008. PMID: 18614796 Free PMC article. - COPII vesicles can affect the activity of antisense oligonucleotides by facilitating the release of oligonucleotides from endocytic pathways.
Liang XH, Sun H, Nichols JG, Allen N, Wang S, Vickers TA, Shen W, Hsu CW, Crooke ST. Liang XH, et al. Nucleic Acids Res. 2018 Nov 2;46(19):10225-10245. doi: 10.1093/nar/gky841. Nucleic Acids Res. 2018. PMID: 30239896 Free PMC article.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10903204', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10903204/'}\]}
- Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289:444–448 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC2168100', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2168100/'}, {'type': 'PubMed', 'value': '10601335', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10601335/'}\]}
- Alvarez C, Fujita H, Hubbard A, Sztul E (1999) ER to Golgi transport: requirement for p115 at a pre-Golgi VTC stage. J Cell Biol 147:1205–1222 - PMC - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC165101', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC165101/'}, {'type': 'PubMed', 'value': '12802079', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12802079/'}\]}
- Alvarez C, Garcia-Mata R, Brandon E, Sztul E (2003) COPI Recruitment is modulated by a Rab1b-dependent mechanism. Mol Biol Cell 14:2116–2127 - PMC - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11389436', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11389436/'}\]}
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3:531–537 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC1319167', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1319167/'}, {'type': 'PubMed', 'value': '12671686', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12671686/'}\]}
- Antonny B, Gounon P, Schekman R, Orci L (2003) Self-assembly of minimal COPII cages. EMBO Rep 4:419–424 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases