Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties - PubMed (original) (raw)
Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties
Christopher B Raub et al. Biophys J. 2008.
Abstract
Multiphoton microscopy (MPM) holds promise as a noninvasive imaging technique for characterizing collagen structure, and thus mechanical properties, through imaging second harmonic generation (SHG) and two-photon fluorescence in engineered and real connective tissues. Controlling polymerization pH to manipulate collagen gel microstructure, we quantified pore and fiber dimensions using both standard methods and image correlation spectroscopy (ICS) on MPM, scanning electron, and darkfield microscopy images. The latter two techniques are used to confirm microstructural measurements made from MPM images. As polymerization pH increased from 5.5 to 8.5, mean fiber diameter decreased from 3.7 +/- 0.7 microm to 1.6 +/- 0.3 microm, the average pore size decreased from 81.7 +/- 3.7 microm(2) to 7.8 +/- 0.4 microm(2), and the pore area fraction decreased from 56.8% +/- 0.8% to 18.0% +/- 1.3% (measured from SHG images), whereas the storage modulus G' and loss modulus G'', components of the shear modulus, increased approximately 33-fold and approximately 16-fold, respectively. A characteristic length scale measured using ICS, W(ICS), correlates well with the mean fiber diameter from SHG images (R(2) = 0.95). Semiflexible network theory predicts a scaling relationship of the collagen gel storage modulus (G') depending upon mesh size and fiber diameter, which are estimated from SHG images using ICS. We conclude that MPM and ICS are an effective combination to assess bulk mechanical properties of collagen hydrogels in a noninvasive, objective, and systematic fashion and may be useful for specific in vivo applications.
Figures
FIGURE 1
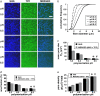
(a) Representative SHG (left column, blue) and TPF (middle column, green) images from collagen hydrogels polymerized at pH 5.5, 6.5, 7.5, and 8.5. Images are 512 × 512 pixels and 230 × 230 _μ_m. n = 12 images per condition were used for microstructure quantification and statistics. Bar represents 50 _μ_m. (b) pH-dependent fiber diameter frequency distributions were constructed from n = 358, 605, 564, and 492 fiber diameter measurements for the pH 5.5, 6.5, 7.5, and 8.5 conditions, respectively. (c) Mean pore size, (d) pore area fraction, and (e) pore density were quantified for each polymerization condition from noise thresholded, inverted SHG, or merged (SHG + TPF) images. Symbols (* and #) indicate statistical significance among polymerization pH groups.
FIGURE 2
(a) Representative SEM images reveal collagen network and fiber characteristics at 20,000× magnification (left column; bar represents 2 _μ_m) and 100,000× magnification (right column; bar represents 500 nm) for the polymerization pH conditions. n = 9, 9, 9, and 6 20,000× images were used for microstructure quantification and statistics. (b) pH-dependent fiber diameter frequency distributions were constructed from n = 477, 513, 629, and 905 fiber diameter measurements for the pH 5.5, 6.5, 7.5, and 8.5 conditions, respectively. (c) Mean pore size, pore area fraction, and pore density were quantified for each polymerization condition from noise-thresholded, inverted SEM images. Symbols (*, #, and §) indicate statistical significance among polymerization pH groups.
FIGURE 3
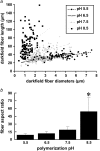
(a) Lengths and diameters of isolated collagen fibers were quantified using ImageJ's line-drawing feature from darkfield images of homogenized collagen hydrogels polymerized at pH 5.5 (•), 6.5 (▵), 7.5 (+), and 8.5 (▪). Each point on the graph represents the length and diameter of one fiber. n = 108 fibers were measured from each polymerization condition. Lines represent linear best fits to the data from each polymerization condition. (b) Mean fiber aspect ratio measured from darkfield images for each pH condition. Error bars represent standard deviation. Symbol (*) indicates statistical significance among pH conditions.
FIGURE 4
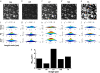
(a) Percentage of colocalizing SHG and TPF pixels, summed from n = 12 paired, noise-subtracted SHG and TPF images per polymerization condition with respect to total SHG and TPF signal-containing pixels. (b) Mask of a representative thresholded TPF images, showing outlines of particles with circularity 0–0.1. (c) Mask of representative thresholded TPF images, showing outlines of particles with circularity 0.5–1. (d) After applying the previous two masks separately to the noise-subtracted TPF image or its SHG counterpart, the mean signal within elliptical particles of circularity 0–0.1 (_SHG_e, _TPF_e) was divided by the mean signal within particles of circularity 0.5–1 (_SHG_c, _TPF_c). Error bars represent standard deviation. (e) in arbitrary units, was estimated for various angles of tilt of the collagen fiber axis with respect to the laser propagation direction, δ: fibers at 10° and 63° of tilt are diagrammed.
FIGURE 5
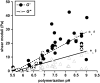
_G_′ and _G_″ of collagen hydrogels, measured by parallel-plate rheometry, vary with polymerization pH. _G_′ (•) and _G_″ (▵ ) were collected at 5% strain and 10 s−1 oscillating shear frequency for n = 48 hydrogels polymerized at pH 5.5–9.0. Lines represent linear best fits to the data for _G_′ and for _G_″. The symbol * indicates that the best fit slopes are significantly different from each other; the symbols # and § indicate that each slope is significantly different from a slope of zero.
FIGURE 6
One 512 × 512 SHG image (a, raw) unprocessed, (b, HP) high-pass filtered, (c, LP) low-pass filtered, (d, NS) noise-subtracted, and (e, Th) thresholded was analyzed with ICS, and (f–j) the ACF, Gaussian fit, and residuals plotted, with the _χ_2 value of the fit reported above the residuals. (k) _W_ICS from each of the Gaussian fits.
FIGURE 7
(a) SEM images were analyzed with ICS, and _W_ICS compared to the geometric mean of hand-measured fiber diameters, d_SEM. x axis error bars represent the 1_σ lower and upper bounds around the geometric mean. (b) Thresholded (Th), noise-subtracted (NS), and unprocessed (RAW) SHG images were analyzed with ICS and by hand measurements; _d_SHG represents the arithmetic mean of hand-measured fiber diameters from SHG images. (c) _W_ICS was calculated as a function of threshold value for SHG images of collagen polymerized at the four pH values. (d) Thresholded, inverted SEM and (e) SHG images were analyzed with ICS, and _P_ICS compared to mean pore size from particle analysis, _P_PA.
FIGURE 8
Scaling relationship for the storage modulus, _G_′, was calculated using rheometric data and ICS, particle analysis, or hand-measured estimates of mesh size and fiber diameter from SHG images. Best-fit slopes and _R_2 values are given in the figure.
Similar articles
- Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy.
Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, Tromberg BJ, George SC. Raub CB, et al. Biophys J. 2007 Mar 15;92(6):2212-22. doi: 10.1529/biophysj.106.097998. Epub 2006 Dec 15. Biophys J. 2007. PMID: 17172303 Free PMC article. - Optical imaging predicts mechanical properties during decellularization of cardiac tissue.
Merna N, Robertson C, La A, George SC. Merna N, et al. Tissue Eng Part C Methods. 2013 Oct;19(10):802-9. doi: 10.1089/ten.TEC.2012.0720. Epub 2013 May 1. Tissue Eng Part C Methods. 2013. PMID: 23469868 Free PMC article. - Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy.
Raub CB, Putnam AJ, Tromberg BJ, George SC. Raub CB, et al. Acta Biomater. 2010 Dec;6(12):4657-65. doi: 10.1016/j.actbio.2010.07.004. Epub 2010 Jul 8. Acta Biomater. 2010. PMID: 20620246 Free PMC article. - Second-harmonic generation imaging of cancer.
Keikhosravi A, Bredfeldt JS, Sagar AK, Eliceiri KW. Keikhosravi A, et al. Methods Cell Biol. 2014;123:531-46. doi: 10.1016/B978-0-12-420138-5.00028-8. Methods Cell Biol. 2014. PMID: 24974046 Review. - Value of multiphoton microscopy in uro-oncology: a narrative review.
Treacy PJ, Khosla A, Kyprianou N, Falagario UG, Tsavaras N, Wiklund P, Tewari AK, Durand M. Treacy PJ, et al. Transl Androl Urol. 2023 Mar 31;12(3):508-518. doi: 10.21037/tau-21-973. Epub 2023 Feb 17. Transl Androl Urol. 2023. PMID: 37032746 Free PMC article. Review.
Cited by
- Degradability tunes ECM stress relaxation and cellular mechanics.
Narasimhan BN, Fraley SI. Narasimhan BN, et al. bioRxiv [Preprint]. 2024 Jul 29:2024.07.28.605514. doi: 10.1101/2024.07.28.605514. bioRxiv. 2024. PMID: 39131364 Free PMC article. Preprint. - Characterization of lamina propria remodeling in pediatric eosinophilic esophagitis using second harmonic generation microscopy.
Haugen EJ, Locke AK, Correa H, Baba JS, Mahadevan-Jansen A, Hiremath G. Haugen EJ, et al. Transl Med Commun. 2024;9(1):10. doi: 10.1186/s41231-024-00170-2. Epub 2024 Mar 22. Transl Med Commun. 2024. PMID: 38698908 Free PMC article. - 3D printing of mechanically functional meniscal tissue equivalents using high concentration extracellular matrix inks.
Wang B, Barceló X, Von Euw S, Kelly DJ. Wang B, et al. Mater Today Bio. 2023 Apr 5;20:100624. doi: 10.1016/j.mtbio.2023.100624. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37122835 Free PMC article. - Effect of Fibrillization pH on Gelation Viscoelasticity and Properties of Biofabricated Dense Collagen Matrices via Gel Aspiration-Ejection.
Rezabeigi E, Griffanti G, Nazhat SN. Rezabeigi E, et al. Int J Mol Sci. 2023 Feb 15;24(4):3889. doi: 10.3390/ijms24043889. Int J Mol Sci. 2023. PMID: 36835306 Free PMC article. - A Beginner's Guide to the Characterization of Hydrogel Microarchitecture for Cellular Applications.
Martinez-Garcia FD, Fischer T, Hayn A, Mierke CT, Burgess JK, Harmsen MC. Martinez-Garcia FD, et al. Gels. 2022 Aug 26;8(9):535. doi: 10.3390/gels8090535. Gels. 2022. PMID: 36135247 Free PMC article. Review.
References
- Comninou, M., and I. V. Yannas. 1976. Dependence of stress-strain nonlinearity of connective tissues on the geometry of collagen fibers. J. Biomech. 9:427–433. - PubMed
- Hiltner, A., J. J. Cassidy, and E. Baer. 1985. Mechanical properties of biological polymers. Ann. Rev. Mater. Sci. 15:455–482.
- Patterson-Kane, J. C., D. A. Parry, H. L. Birch, A. E. Goodship, and E. C. Firth. 1997. An age-related study of morphology and cross-link composition of collagen fibrils in the digital flexor tendons of young thoroughbred horses. Connect. Tissue Res. 36:253–260. - PubMed
- Carroll, E. P., J. S. Janicki, R. Pick, and K. T. Weber. 1989. Myocardial stiffness and reparative fibrosis following coronary embolisation in the rat. Cardiovasc. Res. 23:655–661. - PubMed
- Jalil, J. E., C. W. Doering, J. S. Janicki, R. Pick, W. A. Clark, C. Abrahams, and K. T. Weber. 1988. Structural vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricle. J. Mol. Cell. Cardiol. 20:1179–1187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41RR01192/RR/NCRR NIH HHS/United States
- P41 RR001192-29/RR/NCRR NIH HHS/United States
- R01 HL067954/HL/NHLBI NIH HHS/United States
- P41 RR001192/RR/NCRR NIH HHS/United States
- HL067954/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials