The growth and potential of human antiviral monoclonal antibody therapeutics - PubMed (original) (raw)
Review
The growth and potential of human antiviral monoclonal antibody therapeutics
Wayne A Marasco et al. Nat Biotechnol. 2007 Dec.
Abstract
Monoclonal antibodies (mAbs) have long provided powerful research tools for virologists to understand the mechanisms of virus entry into host cells and of antiviral immunity. Even so, commercial development of human (or humanized) mAbs for the prophylaxis, preemptive and acute treatment of viral infections has been slow. This is surprising, as new antibody discovery tools have increased the speed and precision with which potent neutralizing human antiviral mAbs can be identified. As longstanding barriers to antiviral mAb development, such as antigenic variability of circulating viral strains and the ability of viruses to undergo neutralization escape, are being overcome, deeper insight into the mechanisms of mAb action and engineering of effector functions are also improving the efficacy of antiviral mAbs. These successes, in both industrial and academic laboratories, coupled with ongoing changes in the biomedical and regulatory environments, herald an era when the commercial development of human antiviral mAb therapies will likely surge.
Figures
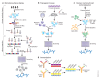
Figure 1. Human antibody techniques.
(a) Phage display exemplifies human antibody library display techniques (phage, bacteria, yeast, mammalian cell and ribosome). Three steps are included in this technique: antibody library construction and display onto the phage surface, selection by panning the library against antigen (Ag) targets, and screening for desired specificity. Diverse human immunoglobulin-variable-region gene segments (as scFv or Fab fragments) are amplified from human B cells of immune or non-immune sources to construct the antibody library. The library is then cloned for display on the surface of the phage. Selection against the desired target is then performed using the phage display library; antibodies that do not bind are washed away and the binders are eluted and amplified by infection of Escherichia coli. After several rounds of such selection, desired specificity can be screened using enzyme-linked immunosorbent assay (ELISA) or techniques such as fluorescent-activated cell sorting (FACS) if a cell-membrane bound protein is the target. Once the desired specificity is obtained, the genes of antibody variable regions can be cloned into whole human IgG expression vectors and transfected into cell lines to produce fully human mAbs (hmAbs). (b) Transgenic mouse. The mouse immunoglobulin genes have been genetically knocked out and replaced with human counterparts. The transgenic mouse will make human antibodies after foreign antigen immunization. The B cells harvested from immunized mice are immortalized by fusion with a myeloma cell line, as in traditional hybridoma technology. The hybridomas are then screened for desired specificity. (c) Memory B-cell immortalization. Memory B cells (CD22+ IgM−, IgD−, IgA−) are isolated from peripheral blood mononuclear cells (PBMCs). They are immortalized by EBV in the presence of a CpG oligodeoxynucleotide and irradiated allogeneic PBMCs. The culture supernatants are then screened directly for specific antibodies. Positive cultures are further cloned by limiting dilution and fully human mAbs can then be produced from the cloned B cells. (d) CDR grafting exemplifies humanization. CDR residues from variable region of a mouse mAb are transferred to human antibody frameworks that have high sequence homology with the mouse counterparts. Katie Ris-Vicari
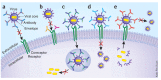
Figure 2. Mechanisms of viral neutralization.
(a) Antibodies block receptor engagement by binding to spikes on an enveloped virus. (b) Antibody blocks virus entry by binding to a viral cellular receptor (or coreceptor) on cell surface. (c) Post-binding/pre-fusion neutralization occurring inside endosome. For some viruses, conformational changes in viral proteins required for fusion are triggered by the low pH in the endosome. MAbs that block the requisite interactions between viral and endosomal membrane proteins would delay or prevent the penetration of the viral core into the target-cell cytoplasm. (d) Post-binding/pre-fusion neutralization occurring at the cell membrane. Antibodies binding to non-receptor binding regions of the viral envelope can neutralize viral infection through interfering with conformational changes that are required for membrane fusion. (e) Inhibition of the release of progeny virus. For example, mAbs to influenza A virion surface neuraminidase prevent the release of virions from the infected cell surface. Not shown in the figure are neutralizing effects of antibodies on the virus before cell binding, which include antibody-mediated virus aggregation to reduce the number of infectious particles. Katie Ris-Vicari
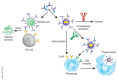
Figure 3. Antiviral mechanisms mediated by antibody Fc.
Antibody-dependent cell-mediated cytotoxicity (ADCC) is shown on the left. Antibody opsonization and activation of the complement leads to antibody-dependent, complement-mediated virolysis, phagocytosis directed against free virions or infected cells (only the free virus is shown) are shown on the right. NK, natural killer. Katie Ris-Vicari
Figure 4. Human mAb combinational therapy.
Two separate neutralizing epitopes that are prevalent on the envelope glycoprotein of a circulating virus are shown. Neutralizing antibody nAb1 and nAb2 can recognize the neutralizing epitope EI (nEI) and EII (nEII) on the circulating strain (row 1). The combination of nAb1 and nAb2 will provide broad neutralization activity and can prevent neutralization escape. A natural variant of nEII or neutralization escape mutant of nAb2 is still recognized by nAb1 (row 2) and nAb4. Likewise, a natural variant of nEI or neutralization escape mutant of nAb1 is still recognized by nAb2 (row 3) and nAb3. Both nAb3 and nAb4 are available and can be used to maintain two nAbs against circulating variant viruses. Only rarely would a combination of nAb3 and nAb4 be required (row 4). Katie Ris-Vicari
Similar articles
- Monoclonal antibodies for prophylactic and therapeutic use against viral infections.
Both L, Banyard AC, van Dolleweerd C, Wright E, Ma JK, Fooks AR. Both L, et al. Vaccine. 2013 Mar 15;31(12):1553-9. doi: 10.1016/j.vaccine.2013.01.025. Epub 2013 Jan 29. Vaccine. 2013. PMID: 23370150 Free PMC article. Review. - Trends in the development and approval of monoclonal antibodies for viral infections.
Reichert JM. Reichert JM. BioDrugs. 2007;21(1):1-7. doi: 10.2165/00063030-200721010-00001. BioDrugs. 2007. PMID: 17263584 Free PMC article. - [Antiviral humanized and human monoclonal antibodies].
Lashkevich VA. Lashkevich VA. Vopr Virusol. 2011 Sep-Oct;56(5):4-8. Vopr Virusol. 2011. PMID: 22171470 Review. Russian. - Monoclonal antibodies for prophylaxis and therapy of infectious diseases.
ter Meulen J. ter Meulen J. Expert Opin Emerg Drugs. 2007 Nov;12(4):525-40. doi: 10.1517/14728214.12.4.525. Expert Opin Emerg Drugs. 2007. PMID: 17979597 Review. - Airway Delivery of Anti-influenza Monoclonal Antibodies Results in Enhanced Antiviral Activities and Enables Broad-Coverage Combination Therapies.
Vigil A, Frias-Staheli N, Carabeo T, Wittekind M. Vigil A, et al. J Virol. 2020 Oct 27;94(22):e00052-20. doi: 10.1128/JVI.00052-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847855 Free PMC article.
Cited by
- Immunoglobulin somatic hypermutation in a defined biochemical system recapitulates affinity maturation and permits antibody optimization.
Jeong SL, Zhang H, Yamaki S, Yang C, McKemy DD, Lieber MR, Pham P, Goodman MF. Jeong SL, et al. Nucleic Acids Res. 2022 Nov 11;50(20):11738-11754. doi: 10.1093/nar/gkac995. Nucleic Acids Res. 2022. PMID: 36321646 Free PMC article. - Nanoscale pathogens treated with nanomaterial-like peptides: a platform technology appropriate for future pandemics.
F Nahhas A, F Nahhas A, J Webster T. F Nahhas A, et al. Nanomedicine (Lond). 2021 Jun;16(14):1237-1254. doi: 10.2217/nnm-2020-0447. Epub 2021 May 14. Nanomedicine (Lond). 2021. PMID: 33988037 Free PMC article. Review. - Infectious disease antibodies for biomedical applications: A mini review of immune antibody phage library repertoire.
Lai JY, Lim TS. Lai JY, et al. Int J Biol Macromol. 2020 Nov 15;163:640-648. doi: 10.1016/j.ijbiomac.2020.06.268. Epub 2020 Jul 8. Int J Biol Macromol. 2020. PMID: 32650013 Free PMC article. Review. - Single Chain Fragment Variable (scFv) Antibodies Targeting the Spike Protein of Porcine Epidemic Diarrhea Virus Provide Protection against Viral Infection in Piglets.
Zhang F, Chen Y, Ke Y, Zhang L, Zhang B, Yang L, Zhu J. Zhang F, et al. Viruses. 2019 Jan 14;11(1):58. doi: 10.3390/v11010058. Viruses. 2019. PMID: 30646521 Free PMC article. - In silico proof of principle of machine learning-based antibody design at unconstrained scale.
Akbar R, Robert PA, Weber CR, Widrich M, Frank R, Pavlović M, Scheffer L, Chernigovskaya M, Snapkov I, Slabodkin A, Mehta BB, Miho E, Lund-Johansen F, Andersen JT, Hochreiter S, Hobæk Haff I, Klambauer G, Sandve GK, Greiff V. Akbar R, et al. MAbs. 2022 Jan-Dec;14(1):2031482. doi: 10.1080/19420862.2022.2031482. MAbs. 2022. PMID: 35377271 Free PMC article.
References
- Good RA, Lorenz E. Historic aspects of intravenous immunoglobulin therapy. Cancer. 1991;68:1415–1421. - PubMed
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004;2:695–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI074518/AI/NIAID NIH HHS/United States
- AI061318/AI/NIAID NIH HHS/United States
- U01 AI061318/AI/NIAID NIH HHS/United States
- U01 AI070343/AI/NIAID NIH HHS/United States
- AI074518/AI/NIAID NIH HHS/United States
- AI060456/AI/NIAID NIH HHS/United States
- AI070343/AI/NIAID NIH HHS/United States
- R01 AI060456/AI/NIAID NIH HHS/United States
- R01 AI052829/AI/NIAID NIH HHS/United States
- AI52829/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials