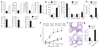Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain - PubMed (original) (raw)
doi: 10.1038/ni1544. Epub 2007 Dec 9.
John T Pesce, Faruk Sheikh, Allen W Cheever, Margaret M Mentink-Kane, Mark S Wilson, Sean Stevens, David M Valenzuela, Andrew J Murphy, George D Yancopoulos, Joseph F Urban Jr, Raymond P Donnelly, Thomas A Wynn
Affiliations
- PMID: 18066066
- PMCID: PMC2692551
- DOI: 10.1038/ni1544
Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain
Thirumalai R Ramalingam et al. Nat Immunol. 2008 Jan.
Abstract
The interleukin 4 receptor (IL-4R) is a central mediator of T helper type 2 (T(H)2)-mediated disease and associates with either the common gamma-chain to form the type I IL-4R or with the IL-13R alpha1 chain (IL-13Ralpha1) to form the type II IL-4R. Here we used Il13ra1-/- mice to characterize the distinct functions of type I and type II IL-4 receptors in vivo. In contrast to Il4ra-/- mice, which have weak T(H)2 responses, Il13ra1-/- mice had exacerbated T(H)2 responses. Il13ra1-/- mice showed much less mortality after infection with Schistosoma mansoni and much more susceptibility to Nippostrongylus brasiliensis. IL-13Ralpha1 was essential for allergen-induced airway hyperreactivity and mucus hypersecretion but not for fibroblast or alternative macrophage activation. Thus, type I and II IL-4 receptors exert distinct effects on immune responses.
Figures
Figure 1
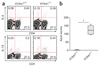
Characterization of _Il13ra1_−/− mice. Flow cytometry of single-cell suspensions of homogenized thymus, spleen and lymph nodes (LN) from naive Il13ra1+/+ and _Il13ra1_−/− littermates. Lymphocytes are gated based on forward- and side-scatter parameters. Numbers in quadrants indicate percent among lymphocytes. Data are representative of two experiments with two to three mice per group.
Figure 2
Macrophages respond to IL-4 but not IL-13 in the absence of type II IL-4 receptor signaling. (a) Immunoprecipitation and immunoblot analysis of STAT6 phosphorylation (pY-STAT6) in Il13ra1+/+ and _Il13ra1_−/− BMDMs stimulated for 30 min at 37 °C with IL-4, IL-13 or IFN-γ (20 ng/ml). (b) Real-time PCR of genes encoding YM1 (Chi3l3) and arginase 1 (Arg1) in BMDMs stimulated for 20 h with IL-4, IL-13 or tumor necrosis factor (TNF; 20 ng/ml), presented as ‘fold increase’ relative to that in unstimulated cells. (c,d) Arginase activity in lysates of BMDMs (c) or thioglycollate-elicited macrophages (d) treated with various concentrations of IL-4 or IL-13 and analyzed after 48 h by measurement of urea production. (e) Nitric oxide production by thioglycollate-elicited macrophages pretreated for 20 h with various concentrations of IL-4 or IL-13, followed by the addition of 200 U IFN-γ (to induce synthesis of inducible nitric oxide synthase); supernatants were analyzed 48 h later for nitrite. Data are representative of two (a,c–b) or four (b) independent experiments with similar results (error bars, s.e.m.).
Figure 3
Serum immunoglobulin production by _Il13ra1_−/− mice chronically infected with S. mansoni. Enzyme-linked immunosorbent assay of SEA-specific immunoglobulin subclasses in serum from naive mice or mice infected with S. mansoni (n = 5–10 mice), collected at 8 and 12 weeks after infection and pooled, presented as absorbance at 405 nm (_A_450). Data from one of two similar experiments (error bars, s.e.m.).
Figure 4
Cytokine production by liver granuloma–associated lymphocytes after S. mansoni infection. Il13ra1+/+ and _Il13ra1_−/− mice were infected with 35 S. mansoni cercariae and were killed at 9 and 12 weeks after infection; leukocytes isolated from perfused livers were stimulated for 3 h with phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A, followed by cytokine-specific antibodies. The frequency of cytokine-producing CD4+ T cells was determined for viable cells, identified by forward- and side-scatter parameters. Each dot represents an individual mouse; horizontal bars in the midst of the dots indicate the mean for each group. *, P < 0.05; **, P < 0.01. Data are representative of three experiments, one on the BALB/c background and two on the C57BL/6 background, which yielded similar results
Figure 5
Attenuated tissue fibrosis in _Il13ra1_−/− mice. Cohorts of Il13ra1+/+ and _Il13ra1_−/− mice were infected with 35 S. mansoni cercariae and were killed at 9 and 12 weeks after infection. (a) Volume of granulomas around viable eggs measured by microscopy of Giemsa-stained sections of paraffin-embedded liver samples. (b) Percent eosinophils among cells constituting the granuloma, assessed in Giemsa-stained sections. (c) Fibrosis, measured as liver hydroxyproline content and normalized to egg numbers. Each dot represents an individual mouse; small horizontal lines indicate the average for each group. Data are representative of three independent experiments and were reproduced on the BALB/c and C57BL/6 backgrounds
Figure 6
Gene expression profiles of Il13ra1+/+ and _Il13ra1_−/− livers after infection with S. mansoni. Real-time PCR of liver mRNA from Il13ra1+/+ and _Il13ra1_−/− mice infected with 35 S. mansoni cercariae and killed at 9 and 12 weeks later; expression is presented as the ‘fold increase’ relative to that in livers of naive mice. (a) Genes encoding cytokines. (b) Genes encoding molecules associated with alternative macrophage activation: mannose receptor (Mrc1), YM1 (Chi3l3), FIZZ1 (Retnla), AMCase (Chia), inducible nitric oxide synthase (Nos2) and arginase 1 (Arg1). (c) Genes encoding molecules involved in extracellular matrix remodeling: TGF-β1 (Tgfb1), MMP9 (Mmp9), procollagen VI (Col6a1) and IL-13Rα1 (Il13ra1). *, P < 0.05. Data are representative of three experiments, one on the BALB/c background and two on the C57BL/6 background, which yielded similar results
Figure 7
Type II IL-4R deficiency protects mice from morbidity after S. mansoni infection. Survival and liver analysis of Il13ra1+/+, _Il13ra1_−/− and _Il13_−/−_Il4_−/− mice (n = 10–12 mice per group) infected with 60 S. mansoni cercariae, monitored up to 22 weeks after infection. (a) Survival curves. (b) Hydroxyproline in infected livers at 22 weeks. (c) Collagen content of infected livers, as assessed by microscopy of picrosirius-stained paraffinembedded liver sections at 22 weeks. Original magnification, ×5. (d) Liver function, assessed by measurement of alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (AP) in serum at 12 weeks after infection. Data are representative of two independent experiments
Figure 8
Impaired expulsion of N. brasiliensis in _Il13ra1_−/− mice. Analysis of Il13ra1+/+ and _Il13ra1_−/− mice (n = 5 mice) inoculated subcutaneously with 500 N. brasiliensis L3 and killed on day 12 after infection. (a) Flow cytometry of intracellular IL-5 and IL-13 in mesenteric lymph node cells stimulated for 3 h ex vivo with phorbol 12-myristate 13-acetate and ionomycin. Density plots are gated on lymphocytes; numbers in quadrants indicate percent cytokine-producing cells among lymphocytes. (b) Adult worm recovery. *, P = 0.0001. Data are representative of two separate experiments.
Figure 9
Protection from allergen-induced airway hyperreactivity in _Il13ra1_−/− mice. Analysis of Il13ra1+/+ and _Il13ra1_−/− mice primed intraperitoneally with SEA on day 0, boosted on day 7, challenged intratracheally with SEA or PBS on days 14,16 and 18, and evaluated on day 19. (a) Expression of mRNA transcripts encoding TH2 cytokines, eotaxin and mucus molecules in lung tissue relative to expression in PBS controls. (b) Total cellularity of bronchoalveolar lavage (BAL) fluid. (c) Composition of lung leukocyte infiltrates, assessed by microscopy of Giemsa-stained sections of paraffin-embedded lung samples, presented as percent of total leukocytes (n = 6 lungs per group). Eos, eosinophils; Ly, lymphocytes; Mac, macrophages. (d) Enzyme-linked immunosorbent assay of total serum IgE. (e) Airway hyperreactivity, as measured by whole-body plethysmography of unrestrained mice exposed to increasing concentration of aerosolized methacholine. Penh, enhanced pause. (f) Mucin staining with Alcian blue–periodic acid Schiff in lungs of SEA-challenged mice. Original magnification, ×10. (g) Mucus production in airway lumen and bronchial walls (airway lining), assigned scores for histology of sections stained with Alcian blue–periodic acid Schiff (PAS+ score; n = 6 mice per group). *, P < 0.05; **, P < 0.01; ***, P < 0.005. Data are from one of two similar experiments with four to seven mice per group.
Similar articles
- The IL-21 receptor augments Th2 effector function and alternative macrophage activation.
Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. Pesce J, et al. J Clin Invest. 2006 Jul;116(7):2044-55. doi: 10.1172/JCI27727. Epub 2006 Jun 15. J Clin Invest. 2006. PMID: 16778988 Free PMC article. - IL-13 receptor α1 differentially regulates aeroallergen-induced lung responses.
Rothenberg ME, Wen T, Shik D, Cole ET, Mingler MM, Munitz A. Rothenberg ME, et al. J Immunol. 2011 Nov 1;187(9):4873-80. doi: 10.4049/jimmunol.1004159. Epub 2011 Sep 28. J Immunol. 2011. PMID: 21957151 Free PMC article. - Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response.
Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson DD, Grusby MJ, Wynn TA. Chiaramonte MG, et al. J Exp Med. 2003 Mar 17;197(6):687-701. doi: 10.1084/jem.20020903. J Exp Med. 2003. PMID: 12642601 Free PMC article. - The Interleukin-13 Receptor-α1 Chain Is Essential for Induction of the Alternative Macrophage Activation Pathway by IL-13 but Not IL-4.
Sheikh F, Dickensheets H, Pedras-Vasconcelos J, Ramalingam T, Helming L, Gordon S, Donnelly RP. Sheikh F, et al. J Innate Immun. 2015;7(5):494-505. doi: 10.1159/000376579. Epub 2015 Mar 7. J Innate Immun. 2015. PMID: 25766112 Free PMC article. - Th2 response polarization during infection with the helminth parasite Schistosoma mansoni.
Pearce EJ, M Kane C, Sun J, J Taylor J, McKee AS, Cervi L. Pearce EJ, et al. Immunol Rev. 2004 Oct;201:117-26. doi: 10.1111/j.0105-2896.2004.00187.x. Immunol Rev. 2004. PMID: 15361236 Review.
Cited by
- IL-4 in the brain: a cytokine to remember.
Gadani SP, Cronk JC, Norris GT, Kipnis J. Gadani SP, et al. J Immunol. 2012 Nov 1;189(9):4213-9. doi: 10.4049/jimmunol.1202246. J Immunol. 2012. PMID: 23087426 Free PMC article. Review. - RGS16 attenuates pulmonary Th2/Th17 inflammatory responses.
Shankar SP, Wilson MS, DiVietro JA, Mentink-Kane MM, Xie Z, Wynn TA, Druey KM. Shankar SP, et al. J Immunol. 2012 Jun 15;188(12):6347-56. doi: 10.4049/jimmunol.1103781. Epub 2012 May 16. J Immunol. 2012. PMID: 22593615 Free PMC article. - Type 2 cytokines: mechanisms and therapeutic strategies.
Wynn TA. Wynn TA. Nat Rev Immunol. 2015 May;15(5):271-82. doi: 10.1038/nri3831. Epub 2015 Apr 17. Nat Rev Immunol. 2015. PMID: 25882242 Review. - Dupilumab (Dupixent®) tends to be an effective therapy for uncontrolled severe chronic rhinosinusitis with nasal polyps: real data of a single-centered, retrospective single-arm longitudinal study from a university hospital in Germany.
Jansen F, Becker B, Eden JK, Breda PC, Hot A, Oqueka T, Betz CS, Hoffmann AS. Jansen F, et al. Eur Arch Otorhinolaryngol. 2023 Apr;280(4):1741-1755. doi: 10.1007/s00405-022-07679-y. Epub 2022 Oct 15. Eur Arch Otorhinolaryngol. 2023. PMID: 36242612 Free PMC article. - The differential expression of IL-4 and IL-13 and its impact on type-2 immunity.
Bao K, Reinhardt RL. Bao K, et al. Cytokine. 2015 Sep;75(1):25-37. doi: 10.1016/j.cyto.2015.05.008. Epub 2015 Jun 11. Cytokine. 2015. PMID: 26073683 Free PMC article. Review.
References
- Finkelman FD, et al. Interleukin-4-and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 2004;201:139–155. - PubMed
- Padilla J, et al. IL-13 regulates the immune response to inhaled antigens. J. Immunol. 2005;174:8097–8105. - PubMed
- Grunig G, et al. Roles of interleukin-13 and interferon-γ in lung inflammation. Chest. 2002;121:88S. - PubMed
- Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases