Is Kir6.1 a subunit of mitoK(ATP)? - PubMed (original) (raw)
Is Kir6.1 a subunit of mitoK(ATP)?
D Brian Foster et al. Biochem Biophys Res Commun. 2008.
Abstract
The subunit composition of the mitochondrial ATP-sensitive K(+)-channel (mitoK(ATP)) is unknown, though some suspect a role for the inward rectifier, Kir6.1, based largely on antibody studies of heart mitochondria. To ascertain the molecular identity of mitoK(ATP) we therefore sought to purify this putative mitochondrial Kir6.1, and conclusively identify the subunits by mass spectrometry. Immunoblots, conducted with two commercially available antibodies, revealed two distinct signals in isolated heart mitochondria, of 51 and 48kDa, respectively. Localization was confirmed by either immuno-gold electron microscopy or by immunofluorescence. Each putative Kir6.1 species was extracted, purified, and identified by LC-MS/MS. The 51kDa band was identified as NADH-dehydrogenase flavoprotein 1, while the preponderant protein in the 48-kDa band was mitochondrial isocitrate dehydrogenase (NADP form). 1D-, 2D-, and native gel analyses were consistent with these assignments. The data suggest it is premature to assign Kir6.1 a role in mitoK(ATP) on the basis of immunoreactivity alone.
Figures
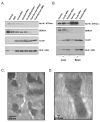
Figure 1. Detection of Kir6.1 Immunoreactivity in Mitochondrial Membranes
Mitochondrial membrane isolation. Kir6.1 immunoreactivity (anti-Kir6.1 (SC)), was detected in bovine (panel A), rat (panel B) mitochondrial membranes. This immunoreactivity paralleled that of CoxIV, an inner membrane marker, even as markers of the plasma membrane (Na+/K+ ATPase) and sarcoplasmic reticulum (SERCA) declined. This trend was also observed in rat tissues, including liver and brain (Panel B). Immuno-electron microscopy. The Kir6.1 (SC) antibody labels mitochondria specifically. Magnification: 40,000 × (Panel C); 100,000 × (Panel D).
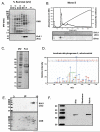
Figure 2. Purification, Characterization and Identification of the 51-kDa Immunoreactive Band
Mitochondrial membranes were solubilized separated by density gradient centrifugation. Kir6.1(SC) antibody recognized a 51kDa protein in fractions containing 30% and 27.5% (w/v) sucrose (Panel A). The fractions were pooled and subjected to FPLC on a Resource Q column eluted with a linear gradient of KCl (Panel B). Fractions containing the 51kDa band were pooled for further analysis. Native gel electrophoresis (Panel C) revealed that the proteins within the fraction migrate as a macromolecular complex with a MW of approx 800 kDa. 2-D gels (IEF/SDS-PAGE; Panel D) show that Kir6.1 immunoreactivity migrates to isoelectric points between 7.5 and 8.4. The 51-kDa Coomassie-stained band that co-migrated with the Kir6.1-immunoreactive band was resolved by SDS-PAGE, excised and processed for MS/MS analysis (Panel E), which identified the predominant protein as NADH-dehydrogenase flavoprotein I. Panel F depicts the MS/MS spectrum of a unique peptide, NACGSGYDFDVFVVR (Mascot ion score: 113, Peptide confidence: 95%, Protein confidence 100%).
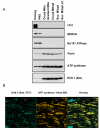
Figure 3. Mitochondrial Kir6.1-Immunoreactivity Revealed by a Second Antibody
Kir6.1 immunoreactivity, assessed with an antibody from Alomone Labs, was detected in bovine mitoplasts (Panel A). Immunoreactivity to Kir6.1 paralleled that of ATP synthase, (beta subunit) through mitochondrial isolation, even as myofilament (cTnI) plasma membrane (Na+/K+ ATPase) and sarcoplasmic reticulum (SERCA) markers declined. Mitochondrial localization of the Kir6.1 signal was confirmed by immunofluorescence microscopy (Panel B). Sections from rat heart ventricles were fixed, prepared and probed as described in the methods section. Kir6.1-immunoreactivity coregisters with that of ATP synthase in sections from bovine ventricle.
Figure 4. Purification, Characterization and Identification the 48-kDa immunoreactive band
Mitochondrial membranes were solubilized and separated by density gradient centrifugation (Panel A). Kir6.1 antibody from Alomone Labs recognized a 48-kDa protein predominantly in fractions containing 17.5% and 15% (w/v) sucrose. The fractions were pooled and subjected to FPLC on a Mono S column eluted with a linear gradient of KCl. (Panel B). Fractions containing the 48-kDa band were pooled for further characterization. The Coomassie-stained band at 48 kDa was resolved, excised and prepared for MS/MS analysis (Panel C). The MS/MS spectrum (Panel D) corresponding to the sequence, DQTNDQVTIDSALATQK (Mascot Score: 135, Peptide confidence: 95%, Protein confidence: 100%) is one of the unique peptides that identifies mitochondrial isocitrate dehydrogenase (NADP-binding form) as the preponderant 48kDa band. The isoelectric point of the Kir6.1 immunoreactivity (pI≈9; Panel E) was consistent with IDH2. Finally, sonication of mitochondria and subsequent centrifugation to separate membrane proteins from those of the matrix (Panel E) revealed that Kir6.1 immunoreactivity was found in the supernatant (matrix) fraction, inconsistent with a bona fide Kir6.1 channel.
Similar articles
- Pharmacological comparison of native mitochondrial K(ATP) channels with molecularly defined surface K(ATP) channels.
Liu Y, Ren G, O'Rourke B, Marbán E, Seharaseyon J. Liu Y, et al. Mol Pharmacol. 2001 Feb;59(2):225-30. Mol Pharmacol. 2001. PMID: 11160857 - Heart mitochondria contain functional ATP-dependent K+ channels.
Lacza Z, Snipes JA, Miller AW, Szabó C, Grover G, Busija DW. Lacza Z, et al. J Mol Cell Cardiol. 2003 Nov;35(11):1339-47. doi: 10.1016/s0022-2828(03)00249-9. J Mol Cell Cardiol. 2003. PMID: 14596790 - Subunit composition of ATP-sensitive potassium channels in mitochondria of rat hearts.
Cuong DV, Kim N, Joo H, Youm JB, Chung JY, Lee Y, Park WS, Kim E, Park YS, Han J. Cuong DV, et al. Mitochondrion. 2005 Apr;5(2):121-33. doi: 10.1016/j.mito.2004.12.001. Mitochondrion. 2005. PMID: 16050978 - [Molecular and functional diversity of ATP-sensitive K+ channels: the pathophysiological roles and potential drug targets].
Nakaya H, Miki T, Seino S, Yamada K, Inagaki N, Suzuki M, Sato T, Yamada M, Matsushita K, Kurachi Y, Arita M. Nakaya H, et al. Nihon Yakurigaku Zasshi. 2003 Sep;122(3):243-50. doi: 10.1254/fpj.122.243. Nihon Yakurigaku Zasshi. 2003. PMID: 12939542 Review. Japanese. - K(ATP) channels "vingt ans après": ATG to PDB to Mechanism.
Babenko AP. Babenko AP. J Mol Cell Cardiol. 2005 Jul;39(1):79-98. doi: 10.1016/j.yjmcc.2004.12.004. Epub 2005 Feb 19. J Mol Cell Cardiol. 2005. PMID: 15978905 Review.
Cited by
- A new pROM king for the mitoK(ATP) dance: ROMK takes the lead.
Rines AK, Bayeva M, Ardehali H. Rines AK, et al. Circ Res. 2012 Aug 3;111(4):392-3. doi: 10.1161/CIRCRESAHA.112.275461. Circ Res. 2012. PMID: 22859667 Free PMC article. No abstract available. - Molecular Bases of Brain Preconditioning.
Deryagin OG, Gavrilova SA, Gainutdinov KL, Golubeva AV, Andrianov VV, Yafarova GG, Buravkov SV, Koshelev VB. Deryagin OG, et al. Front Neurosci. 2017 Jul 25;11:427. doi: 10.3389/fnins.2017.00427. eCollection 2017. Front Neurosci. 2017. PMID: 28790886 Free PMC article. - The cardiac acetyl-lysine proteome.
Foster DB, Liu T, Rucker J, O'Meally RN, Devine LR, Cole RN, O'Rourke B. Foster DB, et al. PLoS One. 2013 Jul 2;8(7):e67513. doi: 10.1371/journal.pone.0067513. Print 2013. PLoS One. 2013. PMID: 23844019 Free PMC article. - mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state.
Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. Cang C, et al. Cell. 2013 Feb 14;152(4):778-790. doi: 10.1016/j.cell.2013.01.023. Epub 2013 Feb 7. Cell. 2013. PMID: 23394946 Free PMC article. - Diabetes mellitus reduces the function and expression of ATP-dependent K⁺ channels in cardiac mitochondria.
Fancher IS, Dick GM, Hollander JM. Fancher IS, et al. Life Sci. 2013 Mar 28;92(11):664-8. doi: 10.1016/j.lfs.2012.11.019. Epub 2012 Dec 19. Life Sci. 2013. PMID: 23261529 Free PMC article.
References
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. 2006;440:470–476. - PubMed
- Lamping K, Gross G. Improved recovery of myocardial segment function following a short coronary occlusion in dogs by nicorandil, a potential new antianginal agent, and nifedipine. J Cardiovasc Pharmacol. 1985;7:158–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources