Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice - PubMed (original) (raw)
Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice
Amanda C Sharko et al. Alcohol Clin Exp Res. 2008 Jan.
Abstract
Background: Emerging evidence implicates metabotropic glutamate receptor (mGluR) function in the neurobiological effects of ethanol. The recent development of subtype specific mGluR antagonists has made it possible to examine the roles of specific mGluRs in biochemical and behavioral responses to ethanol. The purpose of the present study was to determine if mGluRs modulate the acute sedative-hypnotic properties of ethanol in mice.
Methods: C57BL/6J mice were tested for locomotor activity (sedation) and duration of loss of the righting reflex (hypnosis) following acute systemic administration of ethanol alone or in combination with the mGluR5-selective antagonist, 2-methyl-6-(phenylethynyl)pyridine (MPEP), the mGluR1-selective antagonist, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt), or the mGluR2/3-selective antagonist (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495)).
Results: MPEP (10 and 30 mg/kg) significantly enhanced both the sedative and hypnotic effects of ethanol, while LY341495 (10 and 30 mg/kg) significantly reduced the sedative-hypnotic effects of ethanol. CPCCOEt had no effect at any concentration tested. Further loss of righting reflex experiments revealed that LY341495 (30 mg/kg) significantly reduced hypnosis induced by the gamma-aminobutyric acid type A (GABAA) positive modulators, pentobarbital (50 mg/kg) and midazolam (60 mg/kg), and the N-methyl-d-aspartate (NMDA) receptor antagonist, ketamine (150 mg/kg), while MPEP (30 mg/kg) only significantly enhanced the hypnotic properties of ketamine (150 mg/kg).
Conclusions: These findings suggest that specific subtypes of the metabotropic glutamate receptor differentially modulate the sedative-hypnotic properties of ethanol through separate mechanisms of action, potentially involving GABA(A) and NMDA receptors.
Figures
Fig. 1
Effects of mGluR antagonists on loss of righting reflex (LORR). Bars represent the mean (±SEM) duration of ethanol-induced LORR in minutes (n = 6 to 8) following pretreatment with MPEP (A), CPCCOEt (B), or LY341495 (C). *Significantly different from 4 g / kg ethanol alone (p < 0.05, Tukey’s test).
Fig. 2
Effects of mGluR antagonists on total locomotor activity alone and in the presence of a sedative dose of ethanol (A, C, and E). Bars represent the mean (±SEM) horizontal distance traveled in 60 minutes (n = 6 to 8) following pretreatment with vehicle, MPEP (30 mg / kg) (A), LY341495 (30 mg / kg) (C), or CPCCOEt (30 mg / kg) (E) with and without ethanol (2.0 g / kg). *Significantly different from vehicle / vehicle (p < 0.05, Tukey’s test). **Significantly different from vehicle / ethanol (p < 0.05, Tukey’s test). Temporal analysis of mean (±SEM) horizontal distance traveled in 5 minute time intervals (n = 6 to 8) following treatment with vehicle, MPEP (30 mg / kg) (B), LY341495 (30 mg / kg) (D), or CPCCOEt (30 mg / kg) (F) with and without ethanol (2.0 g / kg). *mGluR antagonist / ethanol treatment significantly different from vehicle / ethanol treatment at given time point (p < 0.05, Tukey’s test). *mGluR antagonist / vehicle treatment significantly different from vehicle / vehicle at given time point (p < 0.05, Tukey’s test).
Fig. 3
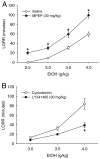
Ethanol-induced loss of righting reflex (LORR) plotted as a function of ethanol dosage, symbols represent the mean (±SEM) duration of LORR in minutes (n = 8) following ethanol with saline pretreatment (open symbols) or in combination with MPEP (A) or LY341495 (B) pretreatment (closed symbols). *Significantly different from vehicle at corresponding dose of ethanol (p < 0.05, Tukey’s test).
Fig. 4
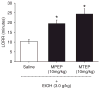
Confirmation of mGluR5 specificity in loss of righting reflex (LOSS). Bars represent mean (±SEM) duration of ethanol-induced LORR in minutes (n = 8) following pretreatment with saline, MPEP, or MTEP. *Significantly different from saline pretreatment (p < 0.05, Tukey’s test).
Similar articles
- Ketamine, but not propofol, anaesthesia is regulated by metabotropic glutamate 5 receptors.
Sou JH, Chan MH, Chen HH. Sou JH, et al. Br J Anaesth. 2006 May;96(5):597-601. doi: 10.1093/bja/ael046. Epub 2006 Mar 10. Br J Anaesth. 2006. PMID: 16531447 - The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice.
Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. Hodge CW, et al. Psychopharmacology (Berl). 2006 Jan;183(4):429-38. doi: 10.1007/s00213-005-0217-y. Epub 2005 Nov 15. Psychopharmacology (Berl). 2006. PMID: 16292590 Free PMC article. - Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions.
Blednov YA, Harris RA. Blednov YA, et al. Int J Neuropsychopharmacol. 2008 Sep;11(6):775-93. doi: 10.1017/S1461145708008584. Epub 2008 Apr 1. Int J Neuropsychopharmacol. 2008. PMID: 18377703 Free PMC article. - Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP.
Lea PM 4th, Faden AI. Lea PM 4th, et al. CNS Drug Rev. 2006 Summer;12(2):149-66. doi: 10.1111/j.1527-3458.2006.00149.x. CNS Drug Rev. 2006. PMID: 16958988 Free PMC article. Review.
Cited by
- Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake.
Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Besheer J, et al. Biol Psychiatry. 2010 May 1;67(9):812-22. doi: 10.1016/j.biopsych.2009.09.016. Epub 2009 Nov 7. Biol Psychiatry. 2010. PMID: 19897175 Free PMC article. - Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking?
Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Goulding SP, et al. Genes Brain Behav. 2011 Feb;10(1):111-26. doi: 10.1111/j.1601-183X.2010.00647.x. Epub 2010 Oct 18. Genes Brain Behav. 2011. PMID: 20807241 Free PMC article. - Adenylyl cyclase-5 in the dorsal striatum function as a molecular switch for the generation of behavioral preferences for cue-directed food choices.
Kim H, Kim TK, Kim JE, Park JY, Lee Y, Kang M, Kim KS, Han PL. Kim H, et al. Mol Brain. 2014 Nov 7;7:77. doi: 10.1186/s13041-014-0077-7. Mol Brain. 2014. PMID: 25378213 Free PMC article. - Glutamate plasticity woven through the progression to alcohol use disorder: a multi-circuit perspective.
Hwa L, Besheer J, Kash T. Hwa L, et al. F1000Res. 2017 Mar 21;6:298. doi: 10.12688/f1000research.9609.1. eCollection 2017. F1000Res. 2017. PMID: 28413623 Free PMC article. Review. - A pilot investigation of the efficacy and safety of magnesium chloride and ethanol as anesthetics in Loligo vulgaris embryos.
Sprecher M, Sprecher SG, Spadavecchia C. Sprecher M, et al. Front Physiol. 2022 Sep 14;13:968047. doi: 10.3389/fphys.2022.968047. eCollection 2022. Front Physiol. 2022. PMID: 36388114 Free PMC article.
References
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Nagren K, Vilkman H, Gustafsson LL, Syvalahti E, Hietala J. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005;182:375–383. - PubMed
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. - PubMed
- Beleslin DB, Djokanovic N, Jovanovic Micic D, Samardzic R. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol. 1997;14:167–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA016629/AA/NIAAA NIH HHS/United States
- P60 AA011605/AA/NIAAA NIH HHS/United States
- AA014983/AA/NIAAA NIH HHS/United States
- R01 AA014983-03/AA/NIAAA NIH HHS/United States
- AA011605/AA/NIAAA NIH HHS/United States
- P50 AA011605/AA/NIAAA NIH HHS/United States
- P60 AA011605-100008/AA/NIAAA NIH HHS/United States
- R01 AA014983/AA/NIAAA NIH HHS/United States
- R37 AA014983/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources