The nucleoporin Nup358 associates with and regulates interphase microtubules - PubMed (original) (raw)
The nucleoporin Nup358 associates with and regulates interphase microtubules
Jomon Joseph et al. FEBS Lett. 2008.
Abstract
The nucleoporin Nup358 resides on the cytoplasmic face of the interphase nuclear pore complex (NPC). During metaphase, its recruitment to kinetochores is important for correct microtubule-kinetochore attachment. Here, we report that a fraction of endogenous Nup358 interacts with interphase microtubules through its N-terminal region (BPN). Cells overexpressing the microtubule targeting domain of Nup358 displayed dramatic alteration in the microtubule organization including increased microtubule bundling and stability. Ectopic expression of BPN and full-length Nup358 exhibited significantly higher levels of acetylated microtubules that were resistant to nocodazole, a microtubule depolymerizing agent. Furthermore, RNAi mediated depletion of Nup358 affected polarized stabilization of microtubules during directed cell migration, confirming the in vivo role of Nup358 in regulating interphase microtubules.
Figures
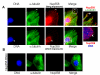
Fig. 1. Nup358 associates with interphase microtubules
(A) CHO-K1 cells were fixed and immunostained for endogenous Nup358 (red) and microtubules (green) using specific antibodies. Arrow indicates cellular extension where Nup358 partially colocalizes with microtubules. The lower panel shows a lower exposure of Nup358 staining, revealing that the bulk of Nup358 is associated with nuclear pores. (B) Cells depleted of Nup358 by RNAi were fixed and stained for endogenous Nup358 (red) and microtubules (green), under conditions identical to those used in Fig. 1A. Image shows a long exposure for anti-Nup358 staining, comparable to the upper panels in Figure 1A. Note that both NPC-associated and cytoplasmic staining of Nup358 are dramatically reduced.
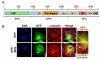
Fig. 2. The N-terminal region targets Nup358 to interphase microtubules
(A) Schematic representation of Nup358 showing different domains. LRR, Leucine-rich region; R, RanBP1-like Ran-GTP binding domain; IR, internal repeats; CHD, Cyclophilin homology domain. The region shown as BPN corresponds to the N-terminal 900 amino acids. (B) GFP-BPN colocalizes with interphase microtubules. COS-7 cells were transfected with GFP or GFP-BPN and 48 hours later were fixed as described in the Materials and Methods section. The cells were stained for microtubules with anti-α-tubulin antibody (red). DNA was stained with Hoechst (blue). Arrow indicates the colocalization of GFP-BPN with microtubules. Note that GFP is distributed in a disperse fashion, and shows no concentration at microtubules. (C) GFP-BPN and GFP-FL-Nup358 shows punctate staining in the cytoplasm that partially colocalizes with microtubules. Cells overexpressing GFP-BPN or GFP-full length Nup358 (GFP-FL-Nup358, green) were fixed and stained for tubulin (red). Arrows and arrow heads indicate punctate staining of GFP-BPN and GFP-FL-Nup358, respectively.
Fig. 2. The N-terminal region targets Nup358 to interphase microtubules
(A) Schematic representation of Nup358 showing different domains. LRR, Leucine-rich region; R, RanBP1-like Ran-GTP binding domain; IR, internal repeats; CHD, Cyclophilin homology domain. The region shown as BPN corresponds to the N-terminal 900 amino acids. (B) GFP-BPN colocalizes with interphase microtubules. COS-7 cells were transfected with GFP or GFP-BPN and 48 hours later were fixed as described in the Materials and Methods section. The cells were stained for microtubules with anti-α-tubulin antibody (red). DNA was stained with Hoechst (blue). Arrow indicates the colocalization of GFP-BPN with microtubules. Note that GFP is distributed in a disperse fashion, and shows no concentration at microtubules. (C) GFP-BPN and GFP-FL-Nup358 shows punctate staining in the cytoplasm that partially colocalizes with microtubules. Cells overexpressing GFP-BPN or GFP-full length Nup358 (GFP-FL-Nup358, green) were fixed and stained for tubulin (red). Arrows and arrow heads indicate punctate staining of GFP-BPN and GFP-FL-Nup358, respectively.
Fig. 3. Ectopic expression of BPN alters microtubule organization and dynamics
(A) COS-7 cells transfected with GFP-BPN (green) were fixed and stained for microtubules (red) using α-tubulin antibodies. Note that the microtubule organization is dramatically affected in GFP-BPN transfected cells (arrow) as compared to untransfected cells (arrow head). DNA (blue) was visualized by Hoechst staining. (B) GFP-BPN transfected cells (green) were fixed and stained for acetylated microtubules (red) using anti-acetyl-α-tubulin antibodies. Note that the GFP-BPN decorating microtubules are highly acetylated. (C) GFP-BPN transfected cells (green) were stained for detyrosinated microtubules (red) using anti-Glu-tubulin antibodies. Note that the GFP-BPN expressing cells have significantly higher levels of Glu-tubulin containing microtubules.
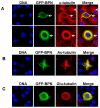
Fig. 4. BPN contains overlapping sequences that mediate Nup358 targeting to nuclear pores and microtubules
(A) Cells expressing low levels of GFP-BPN (green) were fixed and stained for mAb414 antibodies (red), which recognize FxFG containing nucleoporins. DNA was stained with Hoechst (blue). (B) The region corresponding to residues 600-900 of full length Nup358 is sufficient for targeting to the nuclear pore. COS-7 cells were transiently transfected with the indicated constructs, fixed and analyzed for nuclear membrane localization as well as microtubule association by immunofluorescence (IF).
Fig. 5. Ectopic expression of GFP-BPN and GFP-FL-Nup358 increases microtubule stability
GFP, GFP-BPN or GFP-FL-Nup358 (green) overexpressing cells were subjected to nocodazole treatment (10μM, 30 minutes) and later fixed and stained for stable microtubules (red) using anti-acetyl-α-tubulin antibodies. DNA (blue) was visualized by Hoechst staining.
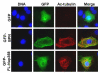
Fig. 6. Nup358 RNAi affects microtubule stability and directional cell migration
CHO-K1 cells were treated with control and Nup358 siRNA and were subjected to wound induced cell migration assay as described in Materials and Methods section. (A) Cells were fixed and stained for total microtubules (green) and detyrosinated microtubules (red) using mouse anti-α-tubulin and rabbit anti-Glu-tubulin antibodies, respectively. DNA was stained with Hoechst (blue). (B) The phase contrast micrograph shows the extent of cell migration into the wound.
Similar articles
- The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo.
Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M. Joseph J, et al. Curr Biol. 2004 Apr 6;14(7):611-7. doi: 10.1016/j.cub.2004.03.031. Curr Biol. 2004. PMID: 15062103 - Nup358 interacts with APC and plays a role in cell polarization.
Murawala P, Tripathi MM, Vyas P, Salunke A, Joseph J. Murawala P, et al. J Cell Sci. 2009 Sep 1;122(Pt 17):3113-22. doi: 10.1242/jcs.037523. Epub 2009 Aug 4. J Cell Sci. 2009. PMID: 19654215 - Nuclear envelope breakdown is coordinated by both Nup358/RanBP2 and Nup153, two nucleoporins with zinc finger modules.
Prunuske AJ, Liu J, Elgort S, Joseph J, Dasso M, Ullman KS. Prunuske AJ, et al. Mol Biol Cell. 2006 Feb;17(2):760-9. doi: 10.1091/mbc.e05-06-0485. Epub 2005 Nov 28. Mol Biol Cell. 2006. PMID: 16314393 Free PMC article. - The dynamic kinetochore-microtubule interface.
Maiato H, DeLuca J, Salmon ED, Earnshaw WC. Maiato H, et al. J Cell Sci. 2004 Nov 1;117(Pt 23):5461-77. doi: 10.1242/jcs.01536. J Cell Sci. 2004. PMID: 15509863 Review. - Molecular Link between DNA Damage Response and Microtubule Dynamics.
Kim JM. Kim JM. Int J Mol Sci. 2022 Jun 23;23(13):6986. doi: 10.3390/ijms23136986. Int J Mol Sci. 2022. PMID: 35805981 Free PMC article. Review.
Cited by
- The low expression of NUP62CL indicates good prognosis and high level of immune infiltration in lung adenocarcinoma.
Ren S, Wang W, Zhang C, Sun Y, Sun M, Wang Y, Zhang X, Lu B, Yao L. Ren S, et al. Cancer Med. 2021 May;10(10):3403-3412. doi: 10.1002/cam4.3877. Epub 2021 May 2. Cancer Med. 2021. PMID: 33934535 Free PMC article. - Dynamics and diverse functions of nuclear pore complex proteins.
Chatel G, Fahrenkrog B. Chatel G, et al. Nucleus. 2012 Mar 1;3(2):162-71. doi: 10.4161/nucl.19674. Epub 2012 Mar 1. Nucleus. 2012. PMID: 22555605 Free PMC article. Review. - Importin-β negatively regulates multiple aspects of mitosis including RANGAP1 recruitment to kinetochores.
Roscioli E, Di Francesco L, Bolognesi A, Giubettini M, Orlando S, Harel A, Schininà ME, Lavia P. Roscioli E, et al. J Cell Biol. 2012 Feb 20;196(4):435-50. doi: 10.1083/jcb.201109104. Epub 2012 Feb 13. J Cell Biol. 2012. PMID: 22331847 Free PMC article. - KIF5B and Nup358 Cooperatively Mediate the Nuclear Import of HIV-1 during Infection.
Dharan A, Talley S, Tripathi A, Mamede JI, Majetschak M, Hope TJ, Campbell EM. Dharan A, et al. PLoS Pathog. 2016 Jun 21;12(6):e1005700. doi: 10.1371/journal.ppat.1005700. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27327622 Free PMC article. - The RanBP2/RanGAP1*SUMO1/Ubc9 complex: a multisubunit E3 ligase at the intersection of sumoylation and the RanGTPase cycle.
Flotho A, Werner A. Flotho A, et al. Nucleus. 2012 Sep-Oct;3(5):429-32. doi: 10.4161/nucl.21980. Epub 2012 Aug 27. Nucleus. 2012. PMID: 22925898 Free PMC article.
References
- Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. - PubMed
- Wu J, Matunis MJ, Kraemer D, Blobel G, Coutavas E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol. Chem. 1995;270:14209–14213. - PubMed
- Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous