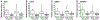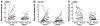Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise - PubMed (original) (raw)
Comparative Study
Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise
Richard A Dennis et al. Physiol Genomics. 2008.
Abstract
The purpose of this investigation was to compare expression of genes that function in inflammation and stress, cell structure and signaling, or remodeling and growth in skeletal muscle of young (32 +/- 7 yr, n = 15) and elderly (72 +/- 5 yr, n = 16) healthy subjects before and after a bout of resistance leg exercises. A real-time RT-PCR method was used to screen 100 transcripts in v. lateralis biopsies obtained before and 72 h postexercise. The screen identified 15 candidates for differential expression due to aging and/or exercise that were measured quantitatively. The median levels of four mRNAs (insulin-like growth factor-1 and its binding protein IGFBP5, ciliary neurotrophic factor, and the metallopeptidase MMP2) were significantly affected by aging and were greater (1.6- to 2.3-fold, P </= 0.05) in the young than elderly muscle at both time points. The median levels of three mRNAs were significantly (P </= 0.05) affected by exercise in the young. The metallopeptidase inhibitor TIMP1 and alpha-cardiac actin mRNAs increased 2-fold and 6.5-fold, respectively, and GDF8 (myostatin) mRNA decreased by 50%. However, elderly muscle did not display any significant changes in gene expression postexercise. Thus, aging muscle shows decreased levels at rest and an impaired response to exercise for a number of mRNAs for factors potentially involved in muscle growth and remodeling. Future studies must determine the functional importance of these gene expression changes to protein synthesis, satellite cell activity, and other processes that are directly involved in the mechanisms of muscle hypertrophy.
Figures
Fig. 1.
The effects of aging on the skeletal muscle transcripts for IGF1 (A), ciliary neurotrophic factor (CNTF, B), matrix metallopeptidase (MMP2, C), and IGF binding protein-5 (IGFBP5, D). Data are presented as box plots denoting the medians (line), the 25th and 75th percentiles (boxes), and the 10th and 90th percentiles (whiskers), along with outliers (dots). Between-group significance, with young being greater than elderly, was seen in both pre (white boxes, P ≤0.05)- and post-resistance (gray boxes, P ≤ 0.05) exercise for each transcript. Expression data were determined by quantitative real-time RT-PCR and normalized to 18s rRNA levels.
Fig. 2.
The effects of exercise on the median (▲) and individual subject expression (line) of transcripts for tissue inhibitor of metalloproteinase (TIMP1, A), α-cardiac actin isoform (ACTC1, B), and growth and differentiation factor-8 (GDF8, C). Post-exercise (Post-ex) expression was significantly different than pre-exercise (Pre-ex) for the young (P ≤ 0.05) but not the elderly for all 3 genes. Expression data were determined by quantitative real-time RT-PCR and normalized to 18s rRNA levels.
Similar articles
- Muscle expression of genes associated with inflammation, growth, and remodeling is strongly correlated in older adults with resistance training outcomes.
Dennis RA, Zhu H, Kortebein PM, Bush HM, Harvey JF, Sullivan DH, Peterson CA. Dennis RA, et al. Physiol Genomics. 2009 Jul 9;38(2):169-75. doi: 10.1152/physiolgenomics.00056.2009. Epub 2009 May 12. Physiol Genomics. 2009. PMID: 19435833 Free PMC article. - Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis.
Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Drummond MJ, et al. J Appl Physiol (1985). 2009 Apr;106(4):1403-11. doi: 10.1152/japplphysiol.90842.2008. Epub 2008 Sep 11. J Appl Physiol (1985). 2009. PMID: 18787087 Free PMC article. - Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle.
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Louis E, et al. J Appl Physiol (1985). 2007 Nov;103(5):1744-51. doi: 10.1152/japplphysiol.00679.2007. Epub 2007 Sep 6. J Appl Physiol (1985). 2007. PMID: 17823296 - Myogenic gene expression at rest and after a bout of resistance exercise in young (18-30 yr) and old (80-89 yr) women.
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Raue U, et al. J Appl Physiol (1985). 2006 Jul;101(1):53-9. doi: 10.1152/japplphysiol.01616.2005. Epub 2006 Apr 6. J Appl Physiol (1985). 2006. PMID: 16601301 - Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle.
Psilander N, Damsgaard R, Pilegaard H. Psilander N, et al. J Appl Physiol (1985). 2003 Sep;95(3):1038-44. doi: 10.1152/japplphysiol.00903.2002. Epub 2003 Apr 25. J Appl Physiol (1985). 2003. PMID: 12716875
Cited by
- Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults.
Ryan AS, Li G, Blumenthal JB, Ortmeyer HK. Ryan AS, et al. Obesity (Silver Spring). 2013 Jul;21(7):1350-6. doi: 10.1002/oby.20216. Epub 2013 May 19. Obesity (Silver Spring). 2013. PMID: 23687104 Free PMC article. - Matrix metalloprotease-3 and tissue inhibitor of metalloprotease-1 mRNA and protein levels are altered in response to traumatic skeletal muscle injury.
Urso ML, Szelenyi ER, Warren GL, Barnes BR. Urso ML, et al. Eur J Appl Physiol. 2010 Jul;109(5):963-72. doi: 10.1007/s00421-010-1435-5. Epub 2010 Mar 27. Eur J Appl Physiol. 2010. PMID: 20349081 - Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training.
Lenk K, Schuler G, Adams V. Lenk K, et al. J Cachexia Sarcopenia Muscle. 2010 Sep;1(1):9-21. doi: 10.1007/s13539-010-0007-1. Epub 2010 Oct 26. J Cachexia Sarcopenia Muscle. 2010. PMID: 21475693 Free PMC article. - Single-Arm Resistance Training Study to Determine the Relationship between Training Outcomes and Muscle Growth Factor mRNAs in Older Adults Consuming Numerous Medications and Supplements.
Dennis RA, Garner KK, Kortebein PM, Parkes CM, Bopp MM, Li S, Padala KP, Padala PR, Sullivan DH. Dennis RA, et al. J Nutr Health Aging. 2018;22(2):269-275. doi: 10.1007/s12603-017-0913-4. J Nutr Health Aging. 2018. PMID: 29380855 - Proteins that accumulate with age in human skeletal-muscle aggregates contribute to declines in muscle mass and function in Caenorhabditis elegans.
Ayyadevara S, Balasubramaniam M, Suri P, Mackintosh SG, Tackett AJ, Sullivan DH, Shmookler Reis RJ, Dennis RA. Ayyadevara S, et al. Aging (Albany NY). 2016 Dec 15;8(12):3486-3497. doi: 10.18632/aging.101141. Aging (Albany NY). 2016. PMID: 27992858 Free PMC article.
References
- Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev 5: 310–331, 2006. - PubMed
- Arking DE, Fallin DM, Fried LP, Li T, Beamer BA, Xue QL, Chakravarti A, Walston J. Variation in the ciliary neurotrophic factor gene and muscle strength in older Caucasian women. J Am Geriatr Soc 54: 823–826, 2006. - PubMed
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/ unloading and the control of muscle mass. Essays Biochem 42: 61–74, 2006. - PubMed
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab 273: E790–E800, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01-RR14288/RR/NCRR NIH HHS/United States
- P01 AG012411/AG/NIA NIH HHS/United States
- P20 RR-16460/RR/NCRR NIH HHS/United States
- P20 RR016460/RR/NCRR NIH HHS/United States
- M01 RR014288/RR/NCRR NIH HHS/United States
- AG012411/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous