DNA transposons and the evolution of eukaryotic genomes - PubMed (original) (raw)
Review
DNA transposons and the evolution of eukaryotic genomes
Cédric Feschotte et al. Annu Rev Genet. 2007.
Abstract
Transposable elements are mobile genetic units that exhibit broad diversity in their structure and transposition mechanisms. Transposable elements occupy a large fraction of many eukaryotic genomes and their movement and accumulation represent a major force shaping the genes and genomes of almost all organisms. This review focuses on DNA-mediated or class 2 transposons and emphasizes how this class of elements is distinguished from other types of mobile elements in terms of their structure, amplification dynamics, and genomic effect. We provide an up-to-date outlook on the diversity and taxonomic distribution of all major types of DNA transposons in eukaryotes, including Helitrons and Mavericks. We discuss some of the evolutionary forces that influence their maintenance and diversification in various genomic environments. Finally, we highlight how the distinctive biological features of DNA transposons have contributed to shape genome architecture and led to the emergence of genetic innovations in different eukaryotic lineages.
Figures
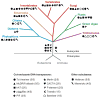
Figure 1
Distribution of the major groups of DNA transposons across the eukaryotic tree of life. The tree depicts 4 of the 5 “supergroups” of eukaryotes (based on Keeling et al. 2005**AU: Please check: 2005 reference is listed only. 2005 is ok) where DNA transposons have been detected. The “unikonts” are represented by the opisthokonts (vertebrates, invertebrates, and fungi) and by the Ameobozoa Entamoeba, the Chromoalveolates by the oomycete Phytophtora infestans, the diatom Thalassiosira pseudonana and several ciliates, the Plantae by the unicellular green algae Chlamydomonas reinhardtii and a broad range of flowering plants, and the Excavates by the parabasalid Trichomonas vaginalis. The occurrence of each superfamily/subclass of DNA transposons is denoted by a different symbol. The data were primarily gathered from the literature (references available upon request). Open symbols denote unpublished observations gathered by the authors or from Repbase (
). The taxonomic breadth of the different groups among the 5 supergroups of eukaryotes is shown in parentheses. These data suggest that 11 of the 12 major types of DNA transposons were already diversified in the common ancestor of eukaryotes.
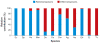
Figure 2
The relative amount of retrotransposons and DNA transposons in diverse eukaryotic genomes. The graph shows the contribution of DNA transposons and retrotransposons in percentage relative to the total number of transposable elements in each species. The data were compiled from papers reporting draft genome sequences (references available upon request) and from the Repeatmasker output tables available at the UCSC Genome Browser (
) or from the following sources: E. histolytica and E. invadens: (159); T. vaginalis: E. Pritham, unpublished data. Species abbreviations: Sc: Saccharomyces cerevisiae; Sp: Schizosaccharomyces pombe; Hs: Homo sapiens; Mm: Mus musculus; Os: Oryza sativa; Ce: Caenorhabditis elegans; Dm: Drosophila melanogaster; Ag: Anopheles gambiae, malaria mosquito; Aa: Aedes aegypti, yellow fever mosquito; Eh: Entamoeba histolytica; Ei: Entamoeba invadens; Tv: Trichomonas vaginalis.
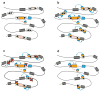
Figure 3. Model for the assembly of a regulatory network by domestication of a transposase and its binding sites
A: Initial transposase domestication event. A family of DNA transposon is shown with autonomous and nonautonomous copies dispersed in the genome. Each TIR (black arrowhead) contains a binding site for a transposase encoded by autonomous copies (pink/yellow boxes). Flanking host genes are shown as grey boxes. One of the transposase genes (yellow box) is recruited. In this example, recruitment is promoted by transcriptional fusion of the transposase to a flanking host gene (blue box) encoding a regulatory domain, leading to the expression of a fusion protein with transpoase (yellow) and regulatory domains (blue). This is similar to the emergence of SETMAR, which arose by fusion of a mariner transposase with an adjacent gene encoding a SET domain. Note however that transposase domestication does not need to involve fusion with another domain, particularly if the transposase itself possesses regulatory activity, as demonstrated for FHY3, a transcription factor in Arabidopsis entirely derived from a Mutator transposase. B: Immediate consequences of transposase domestication. The translational fusion immediately allows the regulatory domain to be tethered to all the sites in the genome recognized by the transposase, i.e. the TIRs of all the transposon copies previously dispersed in the genome. Depending on the genomic environment of the transposons, binding of the fusion protein might have various effects on the expression of the surrounding genes: activation, repression or no effect. These effects are symbolized by the blue arrow acting on adjacent gene. C: Binding sites selection. Natural selection will retain interactions that provide an immediate benefit to the host and will eliminate deleterious interactions. Site elimination (red cross) may occur through substitutions or deletion driven by positive selection. Sites that are selectively neutral (with no positive or negative impact on adjacent genes) are expected to evolve neutrally and most will eventually disappear. Mobility of the transposons at this stage (if it persists) might accelerate the shaping of the network through transposon excision events and/or fixation of new advantageous insertions. D: A regulatory network is born. The end result is the assembly of a regulatory network, where the domesticated transposase and a subset of its ancestral binding sites conferring beneficial interactions are evolving under purifying selection, while the rest of the transposons are eroded by mutations. Note that the system also provides an intuitive opportunity for the establishment of a feedback loop “F” (positive or negative) through domestication of binding sites that were originally linked to the domesticated transposase.
Similar articles
- Helitrons on a roll: eukaryotic rolling-circle transposons.
Kapitonov VV, Jurka J. Kapitonov VV, et al. Trends Genet. 2007 Oct;23(10):521-9. doi: 10.1016/j.tig.2007.08.004. Epub 2007 Sep 11. Trends Genet. 2007. PMID: 17850916 Review. - A Field Guide to Eukaryotic Transposable Elements.
Wells JN, Feschotte C. Wells JN, et al. Annu Rev Genet. 2020 Nov 23;54:539-561. doi: 10.1146/annurev-genet-040620-022145. Epub 2020 Sep 21. Annu Rev Genet. 2020. PMID: 32955944 Free PMC article. Review. - Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses.
Pritham EJ, Putliwala T, Feschotte C. Pritham EJ, et al. Gene. 2007 Apr 1;390(1-2):3-17. doi: 10.1016/j.gene.2006.08.008. Epub 2006 Aug 23. Gene. 2007. PMID: 17034960 - DNA transposons have colonized the genome of the giant virus Pandoravirus salinus.
Sun C, Feschotte C, Wu Z, Mueller RL. Sun C, et al. BMC Biol. 2015 Jun 12;13:38. doi: 10.1186/s12915-015-0145-1. BMC Biol. 2015. PMID: 26067596 Free PMC article. - Transposable elements and factors influencing their success in eukaryotes.
Pritham EJ. Pritham EJ. J Hered. 2009 Sep-Oct;100(5):648-55. doi: 10.1093/jhered/esp065. Epub 2009 Aug 7. J Hered. 2009. PMID: 19666747 Free PMC article. Review.
Cited by
- The SLEEPER genes: a transposase-derived angiosperm-specific gene family.
Knip M, de Pater S, Hooykaas PJ. Knip M, et al. BMC Plant Biol. 2012 Oct 16;12:192. doi: 10.1186/1471-2229-12-192. BMC Plant Biol. 2012. PMID: 23067104 Free PMC article. - Development of chromosomal markers based on next-generation sequencing: the B chromosome of the cichlid fish Astatotilapia latifasciata as a model.
Fantinatti BE, Martins C. Fantinatti BE, et al. BMC Genet. 2016 Aug 18;17(1):119. doi: 10.1186/s12863-016-0427-9. BMC Genet. 2016. PMID: 27539214 Free PMC article. - C-GATE - catalogue of genes affected by transposable elements.
Rebollo R, Farivar S, Mager DL. Rebollo R, et al. Mob DNA. 2012 May 23;3(1):9. doi: 10.1186/1759-8753-3-9. Mob DNA. 2012. PMID: 22621612 Free PMC article. - Evolutionary Dynamics of the Repetitive DNA in the Karyotypes of Pipa carvalhoi and Xenopus tropicalis (Anura, Pipidae).
Zattera ML, Gazolla CB, Soares AA, Gazoni T, Pollet N, Recco-Pimentel SM, Bruschi DP. Zattera ML, et al. Front Genet. 2020 Jul 21;11:637. doi: 10.3389/fgene.2020.00637. eCollection 2020. Front Genet. 2020. PMID: 32793276 Free PMC article. - Genetic Context Significantly Influences the Maintenance and Evolution of Degenerate Pathways.
Bruger EL, Chubiz LM, Rojas Echenique JI, Renshaw CJ, Espericueta NV, Draghi JA, Marx CJ. Bruger EL, et al. Genome Biol Evol. 2021 Jun 8;13(6):evab082. doi: 10.1093/gbe/evab082. Genome Biol Evol. 2021. PMID: 33885815 Free PMC article.
References
- Abrusan G, Krambeck HJ. Competition may determine the diversity of transposable elements. Theor Popul Biol. 2006;70:364–75. - PubMed
- Adams KL, Wendel JF. Novel patterns of gene expression in polyploid plants. Trends Genet. 2005;21:539–43. - PubMed
- Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–51. - PubMed
- Bakre AA, Rawal K, Ramaswamy R, Bhattacharya A, Bhattacharya S. The LINEs and SINEs of Entamoeba histolytica: comparative analysis and genomic distribution. Exp Parasitol. 2005;110:207–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM077582/GM/NIGMS NIH HHS/United States
- R01 GM077582-01A1/GM/NIGMS NIH HHS/United States
- R01 AI 068908-01/AI/NIAID NIH HHS/United States
- R01 GM 77582-01/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases